Background
- Developed as a bridge for cardiac transplant patients, now also being used as a temporary measure in cardiomyopathies and as a final treatment for those not qualifying for transplant
- Shown to prolong survival in these clinical settings (months to years)
- Can have left VAD, right VAD or both ventricles with separate pump
- Goal is to assist heart function and augment cardiac output
- Components:
- Control box: displays battery power, recent alarms
- Pump: Internal pump, taking blood from ventricle and putting into aorta
- Driveline: connects pumps to external battery and controller (usually out of abdomen)
- Power supply: battery packs and a power base station (at home)
- Large console at LVAD centers: shows power, flow, speed, pulsatility
- These are the major variables that will be set by their cardiologists
1st generation: pneumatic pump → pulsatile blood flow
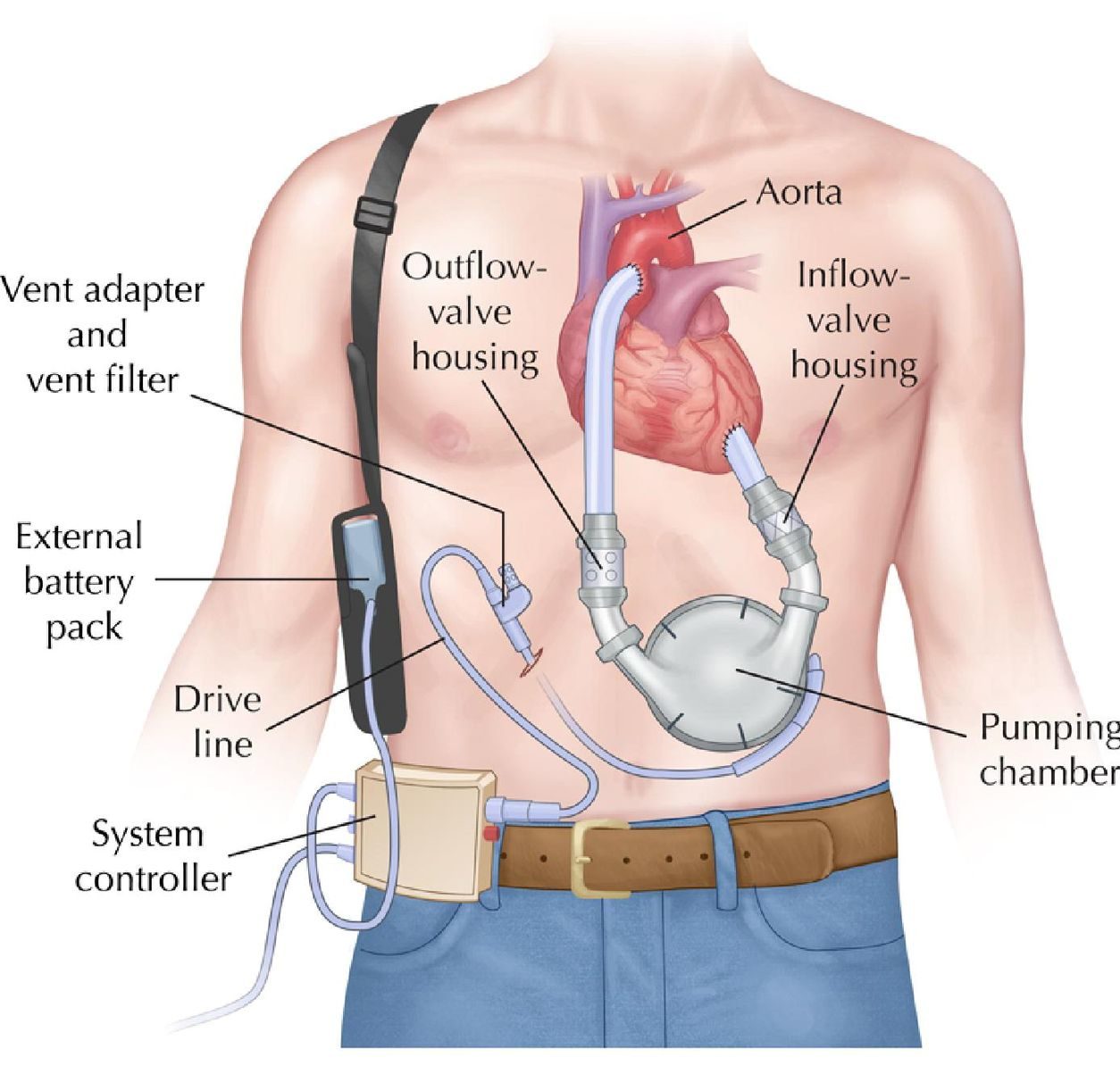
(Wilson 2009)
2nd generation: centrifugal/axial flow pump → continuous flow
- Directly connected to patients LV (lies above the diaphragm within the pericardium)
- Blood back to ascending aorta by separate outflow cannula
- Advantages to 1st generation:
- Smaller size
- Increased durability
- Increased efficiency
- Decreased thrombogenicity
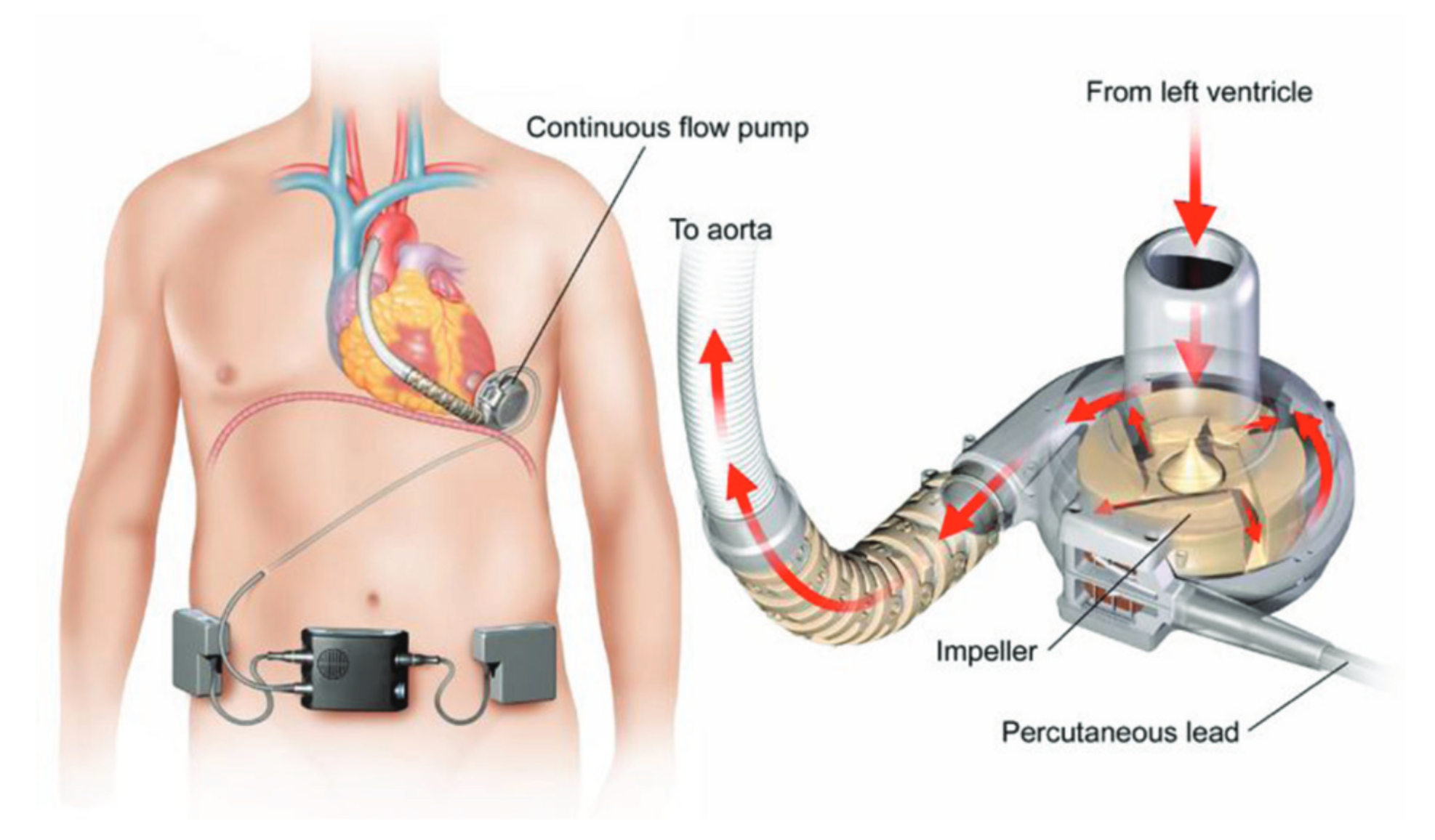
(Aaronson 2012)
General Management
- Contact the LVAD team immediately
Focused History
- Type of device, placement date, previous complications (most devices have this information on it or the patient should know)
Physical exam
- With continuous flow devices, the patient will not have a pulse!
- Blood pressure
- Obtain a manual cuff and arterial doppler device – find brachial or radial artery
- Inflate manual cuff until arterial flow cant be heard, then release; MAP is recorded when arterial flow returns through doppler
- Target MAP Is 70-80 mmHg, max of 90
- Can also monitor with a-line in more critical situations
- Pulse oximetry: may not be accurate if no pulsatile flow – consider obtaining ABG if concerned for respiratory issues
- Cardiac exam: a “hum” should be heard, check for JVD or pulmonary edema (signs there may be right heart failure)
- Power cord: from abdomen usually, evaluate for infection
Diagnostics
- Labs: CBC, CMP, PT/PTT/INR, LDH, haptoglobin, TEG (if available), LDH, BNP, troponin, UA, T&S
- EKG should be performed
- CXR: To evaluate for signs of heart failure, cardiomegaly, pulmonary edema, PNA, driveline damage
- POCUS: LV and RV function, signs of RH strain may signify pulmonary hypertension or a PE. Overdistended LV may indicate pump malfunction, thrombus or severe aortic regurgitation. Check for pericardial effusion or tamponade as well.
Complications and Management
Always consult patients LVAD team!
Bleeding:
- Etiology:
- Usually from supratherapeutic INR (patients are usually on coumadin, antiplatelet therapy with INR goal of 2-3)
- AV malformations
- Acquired von Willebrand disease from pump shear forces
- Signs
- GI bleeding
- Epistaxis
- Intracranial hemorrhage
- Pericardial tamponade /effusion
- Treatment
- Reverse with FFP or PCC (for INR, treat until back to 2-3 to avoid clotting in pump)
- DDAVP or cryoprecipitate (vWD)
- Consider TXA
- Platelet transfusions
- Vitamin K
Infection
- Etiology
- Sepsis
- Pump endocarditis
- Driveline infection
- Signs
- Can result in low flow, hypovolemia
- Most common cause of death (usually within 3 months of placement)
- Treatment
- Antibiotics (cover for MRSA)
- Fluids as needed
- Obtain US/CT to evaluate abscess/collection
Thrombosis
- Can see in 2-35% of patients, suspect in any arrest or cardiogenic shock or decreased flow
- Evaluation
- Knocking sound on cardiac exam can be sign of rotor thrombus
- POCUS: Overdistended LV with shift of septum indicates blockage and can possibly see hypoechoic mass near inflow cannula
- Labs: elevated LDH, hemoglobinuria
- Treatment
- Anticoagulation with a continuous heparin infusion and antiplatelet therapy
- Consider tPA in life threatening situations
Arrhythmia
- Primary – Intrinsic to heart
- Ventricular arrhythmias are common from scarring
- SVT and ventricular tachycardia can be common
- Cardiac output is from the LVAD and not the heart ∴ the patient should have no signs or symptoms if they are VAD-dependent
- Secondary
- Can occur if LV septum/free wall are sucked into conduit vs hypovolemia
- Difficult to distinguish
- Can occur if LV septum/free wall are sucked into conduit vs hypovolemia
- Treatment:
- Prompt fluid challenge
- Emergent bedside echo
- RV failure and reduced LV filling occur if primary arrhythmia not treated
- Manage with cardioversion if unstable or antiarrhythmic meds (amiodarone vs. lidocaine vs beta blockers [no specific choice])
- Use standard ACLS energy recommendations
- Do not place defibrillator pads over the driveline
Stroke
- Risk increases with MAPs >90 (ischemia and hemorrhage)
Pump failure
- Inability to detect MAP
- No sound on exam
- May be due to control box malfunction
- Treatment
- IVF
- Standard ACLS with epinephrine drip, heparin drip
- If suspicion of clot consider VA ECMO early
- If no response after 1 minute of above (fluids, epinephrine) CPR can be initiated (more below)
“Suckdown”
- Occurs when patient’s septum or other part of heart is entrained into inflow cannula
- Etiology
- Decreased LV (from RV failure) volume
- Cardiomyopathies
- Arrhythmias
- Cannula migration
- Hypovolemia
- Tamponade
- Signs
- Hypotension
- Syncope
- Arrhythmias
- Treatment
- Decrease RPM rate
- IVF
Cardiac arrest
- Avoid CPR at all costs if possible, it can theoretically dislodge the LVAD
- Some first generation LVADs have hand pumps that can help with circulation
- Evaluate for other causes of pump failure before doing compressions (i.e. thrombus, battery)
- Check alarms, check the power. Call LVAD team!
- If no flow, no hum, no MAP detectable, and the patient has not responded to any other measures, start ACLS like normal as a last ditch effort (i.e. start compressions and perform CPR)
Take Home Points
- Always contact patient’s LVAD team to assist in care
- Ensure controller is powered on and has backup battery
- BP obtained with doppler, MAP should be between 70-90. Many will not have a pulse.
- If hemodynamically unstable, try fluids first; LVADs are preload sensitive
- If concern for thrombus, use tPA in emergencies
- For cardiac arrests, evaluate for pump failure and then move to ACLS if epinephrine gtt unsuccessful
Resources
- Aaronson KD, Slaughter MS, Miller LW. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation 2012 June 26, 125 (25): 3191-200
- Mancini, D. Practical management of long-term mechanical circulatory devices. UpToDate. Mar, 2018. Link: https://www.uptodate.com/contents/practical-management-of-long-term-mechanical-circulatory-support-devices
- Winters M, et al. Emergency Department Resuscitation of the Critically Ill. Second Edition. Texas: American College of Emergency 2017: p145-154.
- Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med 2007; 357:885-896. PMID: 17761592
- EM:RAP. Episode April 2019, Chapter 9
- Tintinalli, Judith E., et al. Tintinalli’s Emergency Medicine: A Comprehensive Study Guide. Eighth edition. New York: McGraw-Hill Education, 2016: Section 7: 382.
- Sen, A., Larson JS., Kashani, KB., Libricz SL, Alwardt CM, Pajaro O., et al. Mechanical circulatory assist devices: a primer for critical care and emergency physicians. Critical Care, 2016 Jun 25; 20(1): 153. PMID: 27342573
