Background
Fluid resuscitation is a crucial aspect of emergency and critical care. Since the advent of the concept of early goal-directed therapy, we have placed huge emphases on aggressive fluid resuscitation in patients with severe sepsis and septic shock. From EGDT to PROCESS/ARISE/PROMISE to Surviving Sepsis Guidelines, we have seen a shift in how fluid resuscitation is monitored, but the idea of aggressive fluid resuscitation is still the crux of our hemodynamic management of these patients. Yet, the FENICE study showed that in 46 countries, there is a “huge variability in the current practice regarding an FC [fluid challenge]…and may reflect the controversies in current guidelines.” (Cecconi 2015)
This post reviews the critical issues surrounding fluid resuscitation and responsiveness based on this article:
Marik P. Fluid responsiveness and the six guiding principles of fluid resuscitation. Crit Care Med 2016; 44(10): 1920-2. PMID: 26571187
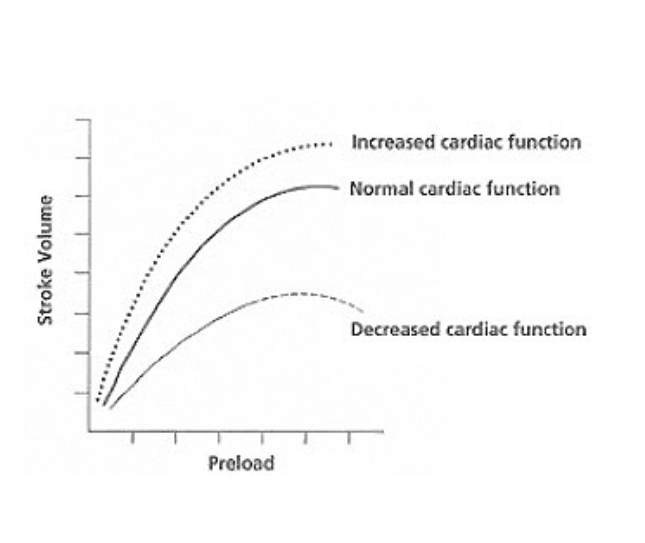
Frank-Starling Curve
Fluid Responsiveness: The foundation of Fluid Resuscitation
- In patients with shock, there is tissue hypoperfusion and decreased cardiac output.
- Fluid administration can be beneficial if it increases stroke volume, and therefore, cardiac output.
- Increased stroke volume leads to increased end-diastolic volume and mean circulating filling pressure, thereby increasing preload and causing volume expansion.
- The goal in shock is to steer patients onto the ascending limb of the Frank Starling curve.
- When fluid administration does not increase stroke volume, there are potential harms.
- Excess fluid administration can lead to tissue edema and ultimately, tissue hypoxia and organ dysfunction.
- Many septic patients have preceding heart failure, about 54% with diastolic dysfunction and 23% with systolic dysfunction, which can be worsened by fluid administration (Landesberg 2012). Over-stretching of the left ventricle (LV) causes impaired LV dilation, leading to pulmonary edema, pulmonary hypertension, and right ventricular dysfunction. In fact, diastolic dysfunction was associated with worse outcomes than systolic dysfunction (Landesberg 2012).
- Therefore, it is critical to ask if the patient’s heart can respond to the additional volume by increasing stroke volume. If it cannot, administration of fluids doesn’t make sense
- A fluid challenge is necessary to determine whether fluid administration will benefit a patient.
- A patient is considered to be fluid responsive if their stroke volume increases by at least 10% after fluid administration (usually 500cc of crystalloids) given as quickly as possible (typically over 10 minutes).
- Only patients who are fluid responsive should receive additional fluids
- Patients who have decreased systolic or diastolic function (on the descending limb of the Frank Starling curve) will not respond to a fluid challenge, even if they are intravascularly depleted.
Clinical Signs, the Chest Radiograph, the CVP, and Ultrasonography Cannot Be Used to Determine Fluid Responsiveness
- Clinical signs can indicate tissue hypoperfusion, but not fluid responsiveness.
- Traditional indicators, such as CVP monitoring and mean arterial pressures (MAP), are not accurate enough.
- Ultrasound is a potential new tool that can be used to assess volume status and fluid responsiveness.
- For volume status, there have been studies looking at IVC caliber in ventilated patients, but this has yet to be validated in spontaneously breathing patients. Lee presented an evidence-informed algorithm based on the available literature to guide fluid resuscitation decisions (Lee 2016).
- For fluid responsiveness, transthoracic measurements of LV outflow tract velocities for the estimation of stroke volume require considerable expertise and not easily or rapidly obtainable.
- Marik suggests alternative methods, such as pulse pressure change measured by radial a-line, LVOT velocity time integral (VTI) on echo, carotid Doppler flow on transesophageal echo, pulse contour analysis, thoracic bioimpedance, bioreactance, etc may be better indicators of fluid responsiveness (Marik 2013).
The PLR Maneuver or a Fluid Challenge Coupled with Real-Time SV Monitoring is the Only Accurate Method to Date for Determining Fluid Responsiveness
- Passive leg raise (PLR) and fluid challenge are two ways to determine fluid responsiveness, especially when used with real-time cardiac output monitors.
- PLR causes a shift in venous blood from the lower extremities to the thoracic compartment; it is essentially an auto-fluid bolus.
- Advantages of PLR
- Reversible
- Non-invasive
- Amount of fluid mobilized is proportional to body size
- Can be used regardless of ventilation mode and cardiac rhythm (Cavallaro 2010)
- Disadvantages of PLR
- Positional changes may be contraindicated
- Less useful in patients with elevated intra-abdominal pressures
- Need to stop other interventions during this maneuver
The Hemodynamic Response to a Fluid Challenge Is Usually Small and Short Lived
- The response to a fluid challenge is usually short lived; within 30 minutes, the cardiac index is usually back to baseline
- The increase in MAP following a fluid challenge is minimal; fluid boluses should not be given if they don’t increase stroke volume and cardiac output
Fluid Responsiveness Does Not Equate to the Need for Fluid Boluses
- Patients should only receive fluid boluses if the hemodynamic benefits are likely to outweigh the risks of becoming overloaded
- As stated previously, the effects of fluid boluses are short-lived and minimal, and continuous fluid resuscitation in these patients will cause fluid overload
- Instead, vasopressors and inotropes should be used early, which will increase organ perfusion while limiting tissue edema
A High CVP Is a Major Factor Compromising Organ Perfusion
- An organ’s blood flow is determined by the MAP and CVP. Our current guidelines recommend targeting a CVP greater than 8mm Hg
- When the CVP is greater than 8mm Hg, certain organs, like the kidney, develop increased renal pressure leading to decreased renal blood flow and acute kidney injury
Take Home Points
- Fluid resuscitation is an essential skill in EM and critical care but is poorly understood
- Fluid administration should be guided both by the concepts of fluid responsiveness (dictated by the Frank-Starling curve) and fluid tolerance
- Because it is difficult to know which patients with sepsis will be fluid responsive, small, frequent boluses may be preferred, and early vasopressor use should be considered
- This may difficult to apply in EM as we do not have technologically advanced cardiac output monitoring devices. However, the principles of fluid resuscitation are sound, and should make us think critically on how to manage these patients
“…in almost half of the patients no hemodynamic variable was used to predict fluid responsiveness – and if used, CVP was used most often.” (Cecconi 2015). Only about 50% of hemodynamically unstable patients are fluid responsive, and only about 50% of those patients are tested for fluid responsiveness; this is a major aspect of emergency and critical care medicine that we can focus on improving.
References
Cavallaro F et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systemic review and meta-analysis of clinical studies. Intensive Care Med. 2010:36(9):1475-83. PMID: 20502865.
Cecconi M et al. Fluid challenges in intensive care: the FENICE study: A global inception cohort study. Intensive Care Med. 2015:41(9):1529-37. PMID: 26162676.
Landesberg G et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012:33(7):895-903. PMID: 21911341.
Lee CV et al. Development of a fluid resuscitation protocol using inferior vena cava and lung ultrasound. J Crit Care. 2016:31(1):96-100. PMID: 26475100.
Marik PE. Noninvasive cardiac output monitors: a state-of the-art review. Cardiothorac Vasc Anesth. 2013:27(1):121-34. PMID: 22609340.
I agree with this article. I definantly agree that fluid resuscitation is nessissary however you do have to make sure the patient can handle it prior to administration. I also like the way you used PLR.
While I do agree with you that fluid responsiveness is essential to fluid administration, a fluid bolus is sometimes essential in EMS. Most protocols require that you “fill the tank” prior to administration of vasopressors. In recent times they have reduced the initial amount of fluid resuscitation (from 1,000mls to 250-500mls) which has helped with over-zealous medics who tend to pressure fluids in to any patient who is hypotensive. In EMS time is never on our side. They want our scene time to be less than 10 minutes on critical calls, therefore IVs and all interventions must be started enroute. Many times we only have 15-20 minute transport times. During this short window we also have to get patient demographics, history and call report prior to arrival. This all takes more time than most people think. We want to do anything we can to help the patient so the first line of treatment is usually fluids. As far as PLR, it looks and sounds great in theory but it isn’t very practical in the field. The time that it takes to see if PLR is working we are bouncing down the road, the monitor isn’t reading properly and before we know it it is time to call report. I think the essential tool is obtaining a good patient history, having good assessment tools and paying attention to your patient. Reassess lung sounds frequently, look at BP, HR, RR ect. Is the patient responding well to interventions? Unfortunately, by the time this is determined we are rolling into the ER…
Christie Dunn