Definition: Chronic Myelogenous Leukemia (CML) is a type of hematopoietic neoplasm involving precursors cells committed to the myeloid line of cellular development (granulocytic, monocytic, erythroid or megakaryocytic elements). Contrary to acute myelogenous leukemia (AML), CML tends to develop more insidiously.
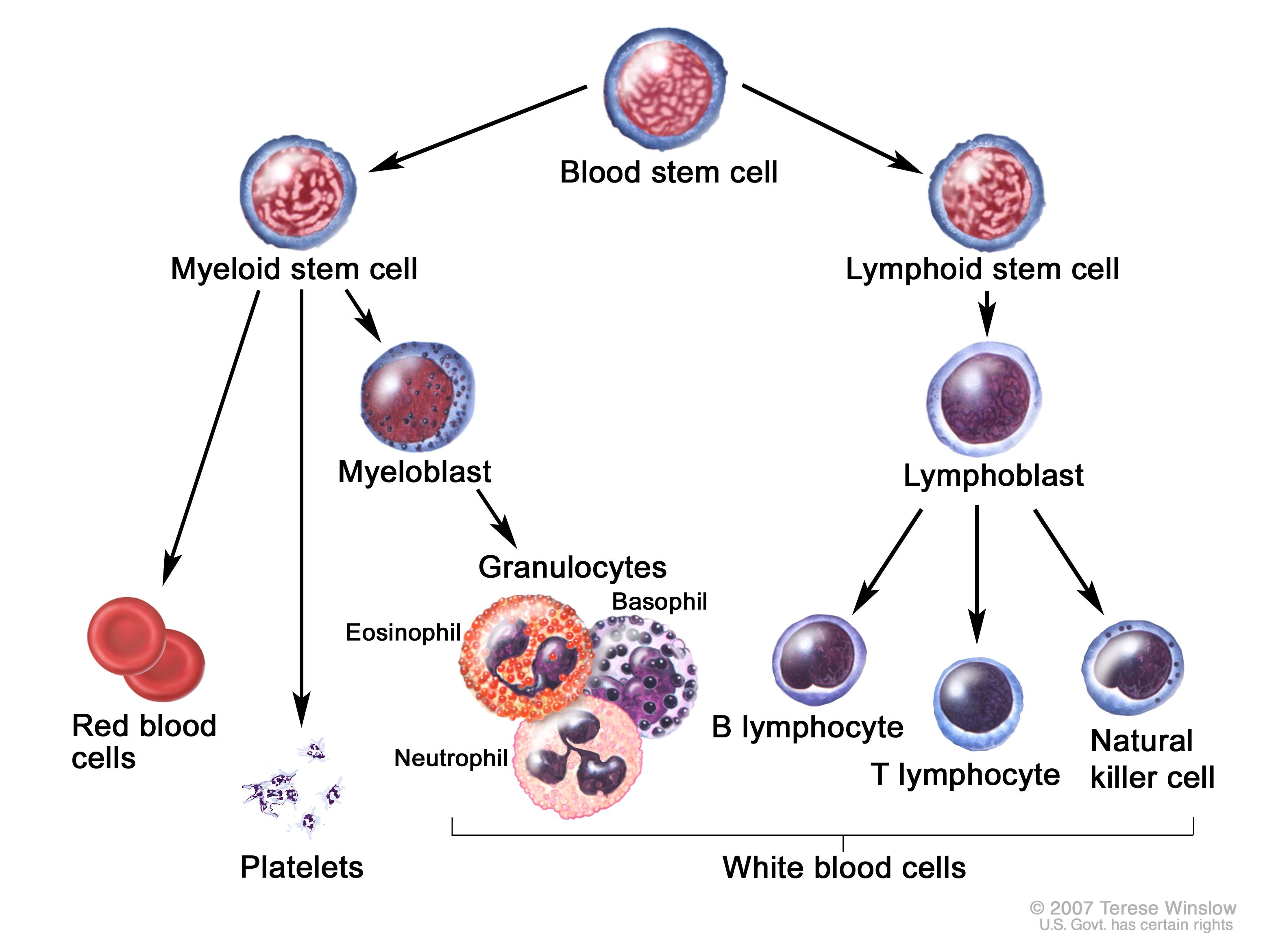
Myelogenous Leukemia (National Cancer Institute www.cancer.gov)
CML exists in three distinct phases depending on the percentage of circulating immature blast cells
| Chronic Myelogenous Leukemia (CML) | ||
| Chronic | Accelerated | Blast |
| < 10% blasts in blood or bone marrow samples | 10% – 20% blasts in blood or bone marrow samples High blood basophil count (>20% of WBC) High WBC refractory to treatment Thrombocytopenia/Thrombocytosis not secondary to treatment New chromosome changes in leukemia cells |
> 20% blasts in blood or bone marrow samples |
Adapted from American Cancer Society
Blast Crisis
- Life-threatening hematologic emergency
- Increase in blast cells in the marrow results in blood hyperviscosity and relative reduction of other cell lines
Epidemiology:
- CML accounts for approximately 15 to 20 percent of leukemias in adults with median age at presentation of 50 (Miller 2016)
- Approximately 90% of CML patients will remain in the chronic phase. 10% will progress to accelerated and then ultimately blast phase.
- The median survival of patients with blast crisis without treatment is 3 months (Shet 2002)
- Risk factors for myelogenous leukemia includes chromosomal abnormalities, ionizing radiation, chemicals, alkylating agents, cigarette smoking, and immunodeficiency diseases. (Marcucci 2005)
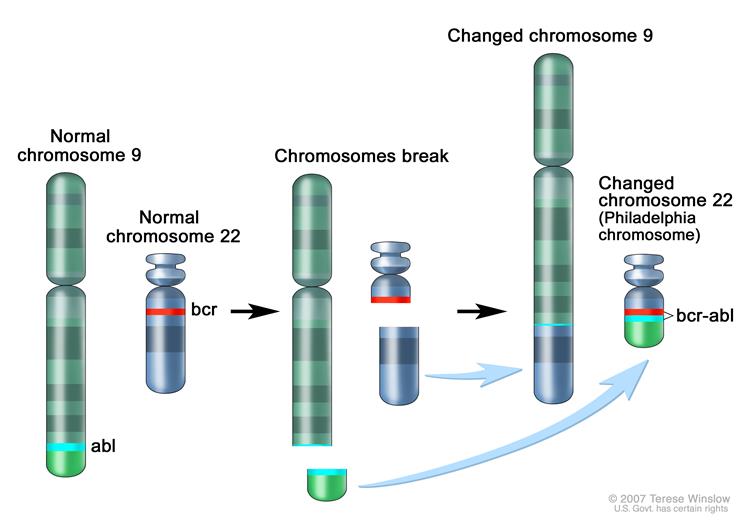
Philadelphia Chromosome (www.cancer.gov)
Pathophysiology
- CML results from an acquired genetic abnormality causing the formation of the BCR-ABL proto-oncogene. This is most commonly the result of a translocation between chromosomes 9 and 22, creating an altered chromosome 22, known as the Philadelphia Chromosome.
- Expression of the BCR-ABL oncogene leads to an unstable genome within the hematopoietic stem cell. This leads to increased proliferation of immature and nonfunctional myelogenous cells.
- Blast Crisis: A critical increase in circulating leukocytes, or leukostasis, leads to blood hyperviscosity and ultimately decreased tissue perfusion via the formation of white cell plugs in the microvasculature.
- Leukostasis is generally defined as a leukocytosis greater than 50 x 109/L or 100 x 109/L
Clinical Presentation
- The majority of patients in blast crisis will often present with signs and symptoms related to pancytopenia (anemia, neutropenia, thrombocytopenia).
- Expansion of the myeloid compartment with immature precursor cells effectively “crowds out” other cell lines leading to a relative anemia and thrombocytopenia.
- Functional Neutropenia (Herlman 2012)
- Numeric increase in leukocytes but cells are not functional
- Loss of function results from genetic abnormalities of cell lines
- Symptoms are vague
- Weakness
- Fatigue
- Night sweats
- Weight loss
- Fever
- Bone pain
- Abdominal fullness
- Hyperviscosity
- Diagnosis based on symptoms, not on WBC count
- Caused by leukocytosis (> 50-100 WBC X 109) leading to leukostasis
- Results in decreased tissue perfusion
- Central Nervous System
- Reduced cerebral blood flow can lead to stroke-like symptoms
- Visual changes, headache, dizziness, tinnitus, gait instability and confusion (Porcu 2000).
- Cardiopulmonary
- Acute respiratory failure
- Congestive heart failure
- Myocardial infarction.
- Additional end organ damage: renal insufficiency, priapism, limb ischemia, and bowel infarction are also known complications (Porcu 2000)
- Central Nervous System
- The most common cause of death in patients in blast crisis is infection secondary to functional neutropenia, followed by fatal hemorrhage secondary to thrombocytopenia (Cervantes 1992)
ED Approach and Management
- Aggressively treat any signs of infection with broad spectrum antibiotics
- 80% of patients with leukostasis present with fever.
- It is unknown if this is secondary to local inflammation or concurrent infection. (Porcu 2000)
- Anemia should be treated cautiously
- Administration of packed red blood cells and use of diuretics can actually worsen blood viscosity and leukostasis (Schiffer 2016)
- Life threatening hemorrhage should be treated with resuscitation and products as needed
- If leukostasis is suspected by clinical findings, emergent consult to Hematology for leukopheresis is indicated. (Bruserud 2013)
Take Away Points
- The most common complications of patients in CML blast crisis are anemia, thrombocytopenia associated bleeding, and infections secondary to functional neutropenia
- If the patient has symptoms concerning for leukostasis and hyper viscosity (CNS changes, renal dysfunction, cardiovascular manifestations etc), emergent consult to Hematology should be made for possible leukopheresis.
Read More
emDocs: Blast Crisis: ED-Focused Managementent
Up to Date: Hyperleukocytosis and leukostasis in hematologic malignancies
Bruserud O et al. Hyperleukocytosis and leukocytapheresis in acute leukaemias: experience from a single centre and review of the literature of leukocytapheresis in acute myeloid leukaemia. Transfus Med. 2013; 23(6): 397-406 PMID: 23919332
Cervantes F et al. Causes of death in chronic myeloid leukemia Sangre 1991; 36 (3): 183-6 PMID: 1948535
Herlmann R. How I treat CML blast crisis. Blood 2012; 120(4): 737-47 PMID: 22563972
Marcucci G et al. Prognostic factors and outcome of core binding factor acute myeloid leukemia patients with t(8;21) differ from those of patients with inv(16): a Cancer and Leuemia Group B study. J Clin Oncol. 2005; 20(23): 5707-17 PMID: 16110030
Miller KD et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016; 66(4): 271-89 PMID: 27253694
Porcu P et al. Hyperleukocytic leukemias and leukostasis: a review of pathophysiology, clinical presentation and management. Leuk Lymphoma. 2000; 39: 1-18 PMID: 10975379
Shet AS et al. Chronic myelogenous leukemia: mechanisms underlying disease progression. Leukemia 2002; 16(8): 1402-11 PMID: 12145676

Good blog post. I absolutely appreciate this site.
Continue the good work!