Background
Status epilepticus is associated with significant morbidity and mortality. Benzodiazepines are recommended as first line agents but their efficacy is approximately 50%. The most commonly recommended 2nd line agents are Phenytoin and Fosphenytoin. Their use is associated with an efficacy of approximately 50%. In addition, their use is associated with significant adverse events. Levetiracetam (Keppra) had been proven efficacious in small case series, can be administered more rapidly (5 minutes vs 20 minutes) and has the potential for fewer adverse events.
Clinical Question
In pediatric patients in convulsive status epilepticus unresponsive to first line therapy with 2 doses of a benzodiazepine, is Levetiracetam superior to Phenytoin as a second line anticonvulsant in improving the rate of seizure cessation 5 minutes after study drug infusion is completed?
Population
Inclusion:
- 3 month to 6 years
- Status epilepticus: International League Against Epilepsy definition
- Unresponsive with continued movements (tonic, jerky) > 5 minutes
- >2 recurrent seizures without a recovery of consciousness between
- >3 seizures in the past hour with current seizure
- Unresponsive to 2 doses of a benzodiazepine (94% received Midazolam)
Exclusion:
- On Levetiracetam or Phenytoin at baseline
- 2nd line AED in past 24 hrs
- History of seizures refractory to Phenytoin
- Status Epilepticus due to major trauma or eclampsia
Setting:
- PREDICT Network (Australia, New Zealand), n = 13 (8 Children’s, 5 General Hospitals), 3/2015-11/2017
Intervention
Intervention:
- Levetiracetam: 40 mg/kg IV/IO (maximum dose 3 grams) over 5 minutes
Co-Intervention:
- At 5 minutes after completion of the infusion, if seizure activity continued the patient received the alternative study drug.
Control
Phenytoin: 20 mg/kg IV/IO (maximum dose 1 gram) over 20 minutes
Outcomes
Primary Outcome:
- Seizure cessation 5 minutes after study drug infusion completed
- 10 minutes after starting Levetiracetam Infusion (5-minute infusion)
- 25 minutes after starting Phenytoin infusion (20-minute infusion)
Secondary Outcomes:
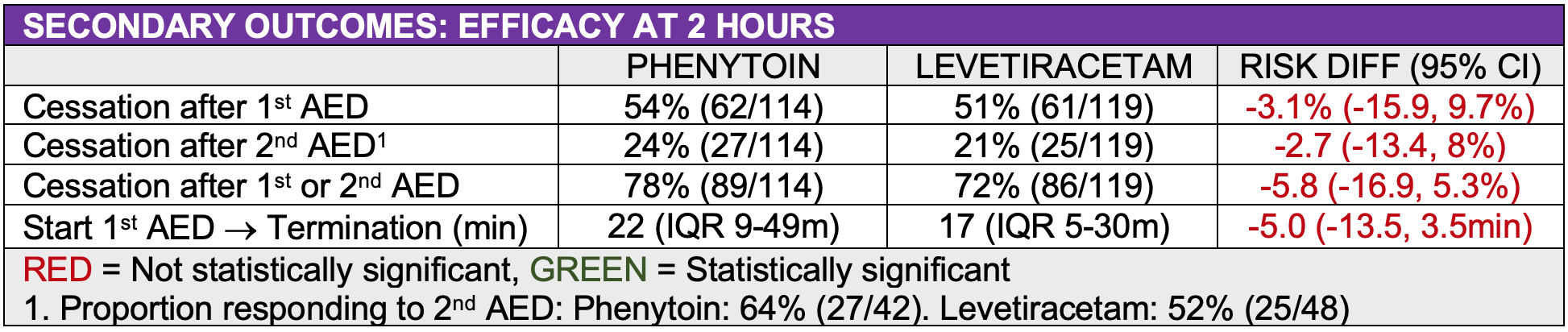
- Seizure cessation at 2 hours after start of infusion without the need for:
- Further seizure management or RSI
- Need for RSI for seizure management
- Time to seizure cessation
- Length of stay: Inpatient, ICU, rate of ICU admission
- Serious adverse events: Death, serious unexpected airway complication in the first 24 hours, cardiovascular instability (Arrest, arrythmias requiring defibrillation), other
- Safety outcomes: Multiple
Design
Interventional: Randomized Clinical Trial (Superiority hypothesis)
Primary Results
N = 233 (Phenytoin 115, Levetiracetam 119)
Efficacy:
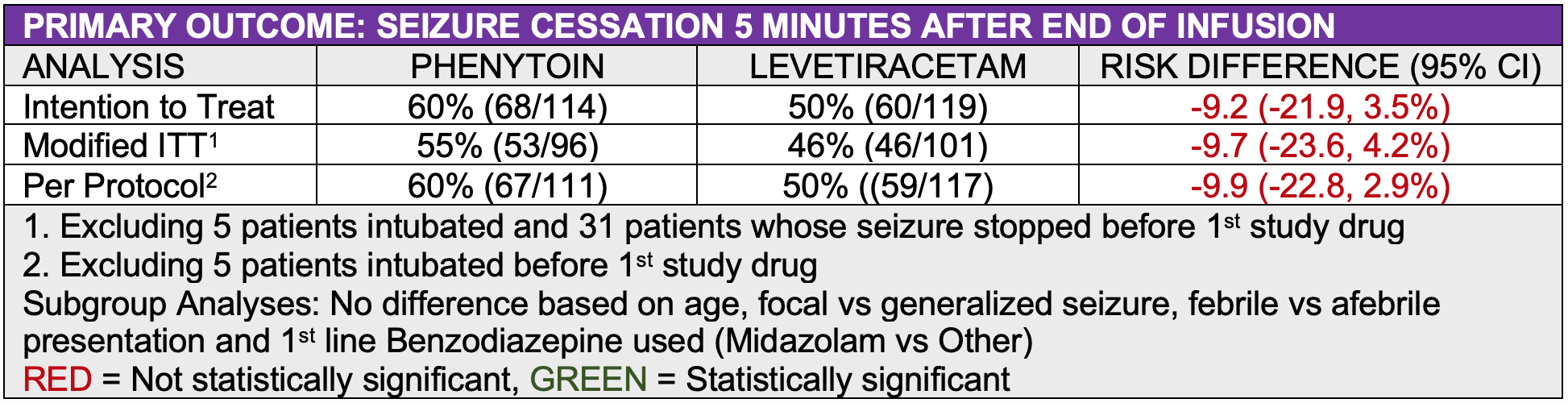
- In the primary intention to treat analysis, there was not a statistically significant difference between the two study medication in the primary outcome of seizure cessation 5 minutes after the completion of the study medication infusion
- Phenytoin: 60% (68/114), Levetiracetam: 50% (60/119)
- Risk Difference: -9.2, 95% CI (-21.9, 3.5)).
- The authors considered a 20% improvement clinically significant
Safety:
- There was no statistically significant difference between the two study medications in any of the secondary safety outcomes analyzed. The sample size is inadequate to assess the likelihood of rare adverse events such as Steven’s Johnson Syndrome.
See “Read More” Section for tables
Strengths
- Multicenter, randomized trial (PREDICT Network: Australia, New Zealand)
- Cross-over design: Received second study medication if first was ineffective
- International League Against Epilepsy definition of Status Epilepticus
- Relevant emergency department outcomes
- Reasonable sample size: N=239
- Included an intention-to-treat, modified intention-to-treat and per protocol analyses
Limitations
- Unblinded
- Excluded patients on Keppra or Phenytoin at baseline
- Superiority rather than non-inferiority hypothesis
- Time to first study medication: 73 minutes
- Sample size inadequate to assess the likelihood of rare adverse events
Author's Conclusions
“In conclusion, we found that levetiracetam is not superior to phenytoin for treatment of children with convulsive status epilepticus with continued clinical seizure activity after treatment with benzodiazepines. Although both drugs were associated with considerable failure rates when given by themselves, treatment with one drug and then the other reduced the failure rate by more than 50%, at the expense of only an additional 10 minutes (compared with giving phenytoin alone). Clinicians should therefore consider sequential use of phenytoin and levetiracetam, or levetiracetam and phenytoin, for management of paediatric convulsive status epilepticus before moving on to RSI and intubation.”
Our Conclusions
While Keppra was not found to be superior the Phenytoin its safety profile and short time of administration makes it an attractive alternative. An additional 22% of patients responded to the alternative drug is the first was not efficacious potentially reducing the rapid sequence intubation rate. Giving both drugs serially should be considered.
Approximately 50% of the patients were still seizing after the first study drug and 25% after the second alternative study drug. This makes it essential to anticipate the need for addition antiepileptic medications and prepare equipment and medications for rapid sequence intubation.
Potential Impact To Current Practice
- Consider Keppra as 2nd line antiepileptic
- Consider giving Keppra and Phenytoin serially to prevent RSI
Read More
SEE ALSO
Lyttle MD, Rainford NEA, Gamble C, Messahel S, Humphreys A, Hickey H, Woolfall K, Roper L, Noblet J, Lee ED, Potter S, Tate P, Iyer A, Evans V, Appleton RE; Paediatric Emergency Research in the United Kingdom & Ireland (PERUKI) collaborative.
Levetiracetam Versus Phenytoin For Second-Line Treatment Of Paediatric Convulsive Status Epilepticus (EcLiPSE): A Multicentre, Open-Label, Randomised Trial.
Lancet. 2019 Apr 17. pii: S0140-6736(19)30724-X., PMID: 31005385
LINKS
PEMCAR – Keppra vs Phenytoin as 2nd Line AED (CONSEPT Trial from PREDICT) – Lancet 2019
