Background
Cellulitis is a common emergency department (ED) presentation. Despite the fact that diagnosis remains relatively straight forward, complexity remains in management in terms of the causative agent and appropriate antibiotic regimen. Though beta-hemolytic Streptococci are the most common causative agents there is increasing prevalence of community acquired methicillin-resistant Staphylococcus aureus (MRSA). Cephalexin has long been used to treat uncomplicated cellulitis because of it’s activity against streptococci and methicillin-sensitive S. aureus (MSSA). Despite the current Infectious Disease Society of America (IDSA) recommendations against routine coverage of MRSA, trimethoprim-sulfamethoxazole (TMP-SMX) is often added to cephalexin (Stephens 2014). While there are other single options for coverage, they either have suboptimal MRSA coverage (i.e. clindamycin and doxycycline) or are more expensive (i.e. linezolid). Without reliable ways to determine which patients need MRSA coverage, it is unclear which patients with uncomplicated cellulitis need to be discharged with MRSA coverage and which will do fine with a single agent.
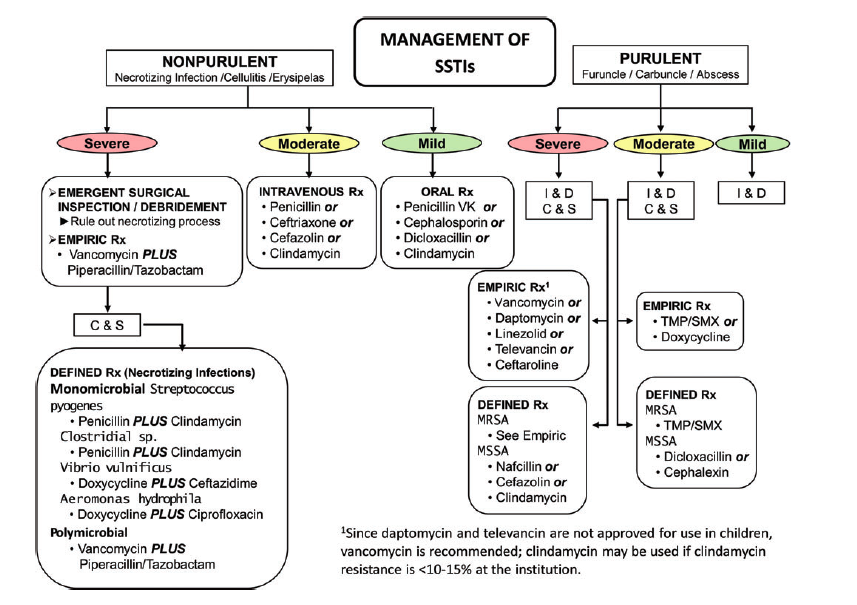
SSTI Flow Diagram (Stevens 2014)
Clinical Question
Does combination therapy of cephalexin plus TMP-SMX result in higher clinical cure rates than cephalexin alone in the treatment of uncomplicated cellulitis?
Population
Patients > 12 years of age with uncomplicated cellulitis (erythema without abscess, purulent drainage or associated wound) with symptoms < 1 week in duration and at least 2.0 cm diameter of cellulitis.
Intervention
Cephalexin 500 mg Q6 X 7 days plus TMP-SMX 320mg/1600mg Q12 X 7 days
Control
Cephalexin 500 mg Q6 X 7 days plus placebo
Outcomes
Primary: 25%, fever, swelling or tenderness at 3-4 day follow up, no decrease in erythema, swelling or tenderness at 8-10 day follow up or anything more than minimal erythema, swelling or tenderness at 14-21 day follow up.
Secondary:
Composite clinical cure rate (resolution of all symptoms and signs), surgical drainage procedures, changes in erythema size, presence of swelling/induration and tenderness, invasive infections, skin infections at the same or different site, hospitalizations, similar infections in household contacts, days missed from normal activities and days of analgesic use
Design
Multicenter, randomized, double-blind, placebo controlled superiority trial. Clinically significant difference defined as > 10% difference
Excluded
Patients with an associated underlying skin condition in the same area as the cellulitis, history of IV drug use with fever, concurrent infection at another site, immunosuppression, indwelling device, suspected osteomyelitis or septic arthritis, diabetic foot ulcer, decubitus ulcer, ischemic ulcer, mammalian bite, wound with organic foreign body, infection of another organ system/site, long-term care residence, incarceration, creatinine clearance <50mL/min, allergy or intolerance to cephalosporins or trimethoprim-sulfamethoxazole, taking warfarin, phenytoin, methotrexate or pregnant/lactating.
Primary Results
- Performed both per-protocol (n = 496) and intention-to-treat (n = 411) analysis
- 500 patients enrolled
- Determined to give 90% power to detect a 10% difference
- Intervention group n = 248 (49.6%)
- Control group n = 248 (49.6%)
- 4 patients excluded prior to treatment initiation
- Per-protocol analysis
- 411/496 (82.2%) eligible for per-protocol analysis
- Intervention group n = 218 (87.9%)
- Control group n = 193 (77.8%)
- 100% adherent with therapy: 51.8% (257/496)
Critical Results
|
Cephalexin + TMP-SMX |
Cephalexin + Plaebo |
Difference |
|
|
Per-Protocol Clinical Cure |
83.5% (182/218) |
85.5% (165/193) |
-2.0% (CI -9.7 – 5.7% p = 0.50) |
|
Modified Intention-to-Treat Clinical Cure |
76.2% (189/248) |
69.0% (171/248) |
7.3% (CI -1.0 – 15.5% p = .07) |
Strengths
- Multicenter nature increases external validity
- Broad inclusion criteria
- Follow-up evaluations occurred in person or if unable, by telephone
- Randomization and blinding appropriately performed
- Patient centered primary outcome
- Performed both per-protocol and intention-to-treat analysis
- Patients lost to follow up were considered treatment failure which would underestimate the results of this trial for clinical cure
Limitations
- Enrollment just short of goal number (n = 496)
- Study powered to find 10% difference but smaller differences may still be clinically significant
- US performed on every patient to eliminate abscess. This is not standard practice in many places and may reduce external applicability
- In the per-protocol group, the cephalexin + placebo group had a considerably lower adherence rate (i.e taking <75% of antimicrobial therapy, missing follow-up visits, or other protocol deviations). Unblinding is a possible issue
Other Issues
- Per protocol analysis improves the accuracy of the results of clinical response, however a per-protocol analysis may not reflect what actually happens in the clinical practice
Author's Conclusions
”Among patients with uncomplicated cellulitis, the use of cephalexin plus trimethoprim-sulfamethoxazole compared to cephalexin alone did not result in higher rates of clinical resolution of cellulitis in the per-protocol analysis. However, because imprecision around the findings in the modified intention-to-treat analysis included a clinically important difference favoring cephalexin plus trimethoprim-sulfamethoxazole, further research may be needed.”
Our Conclusions
In patients with cellulitis and no evidence of abscess who are well appearing without signs of systemic infection, it is reasonable to treat with antibiotics covering streptococci and not adding coverage for MRSA initially (i.e. cephalexin alone). Patients should be given follow up and good instructions for return visit for treatment failure.
Potential Impact To Current Practice
These results may influence providers to avoid the routine prescription of TMP-SMX in patients with uncomplicated cellulitis and stick to an anti-streptococci medication alone.
Bottom Line
Routine addition of TMP-SMX to cephalexin in patients with uncomplicated cellulitis did not result in an increased clinical cure rate. Based on the best available evidence, providers should follow the IDSA recommendations for initial treatment.
Read More
EM Lit of Note: Double Coverage, Cellulitis Edition
Pharm ER Tox Guy: Uncomplicated Cellulitis? Consider Strep-Only Coverage
Core EM: Cellulitis
References
Stevens DL et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the infectious diseases society of America. Clin Infect Dis 2014; 59(2): e10-52. PMID: 24973422

Indeed a great and purposeful article!