Background:
- An update to our 2015 post on HIET for beta-adrenergic receptor and calcium channel antagonists overdose.
- Beta-adrenergic receptor antagonists (Beta blockers, BB) and calcium channel antagonists (calcium channel blockers) are common drugs that can produce profound cardiac depression and shock when taken in overdose.
- 2020 National Poison Data System report:
- 10,994 beta blocker overdoses
- 18 deaths
- 6,132 calcium channel blocker overdoses
- 45 deaths
- High case fatality rate, behind only acetaminophen, opiates, and stimulants
- 10,994 beta blocker overdoses
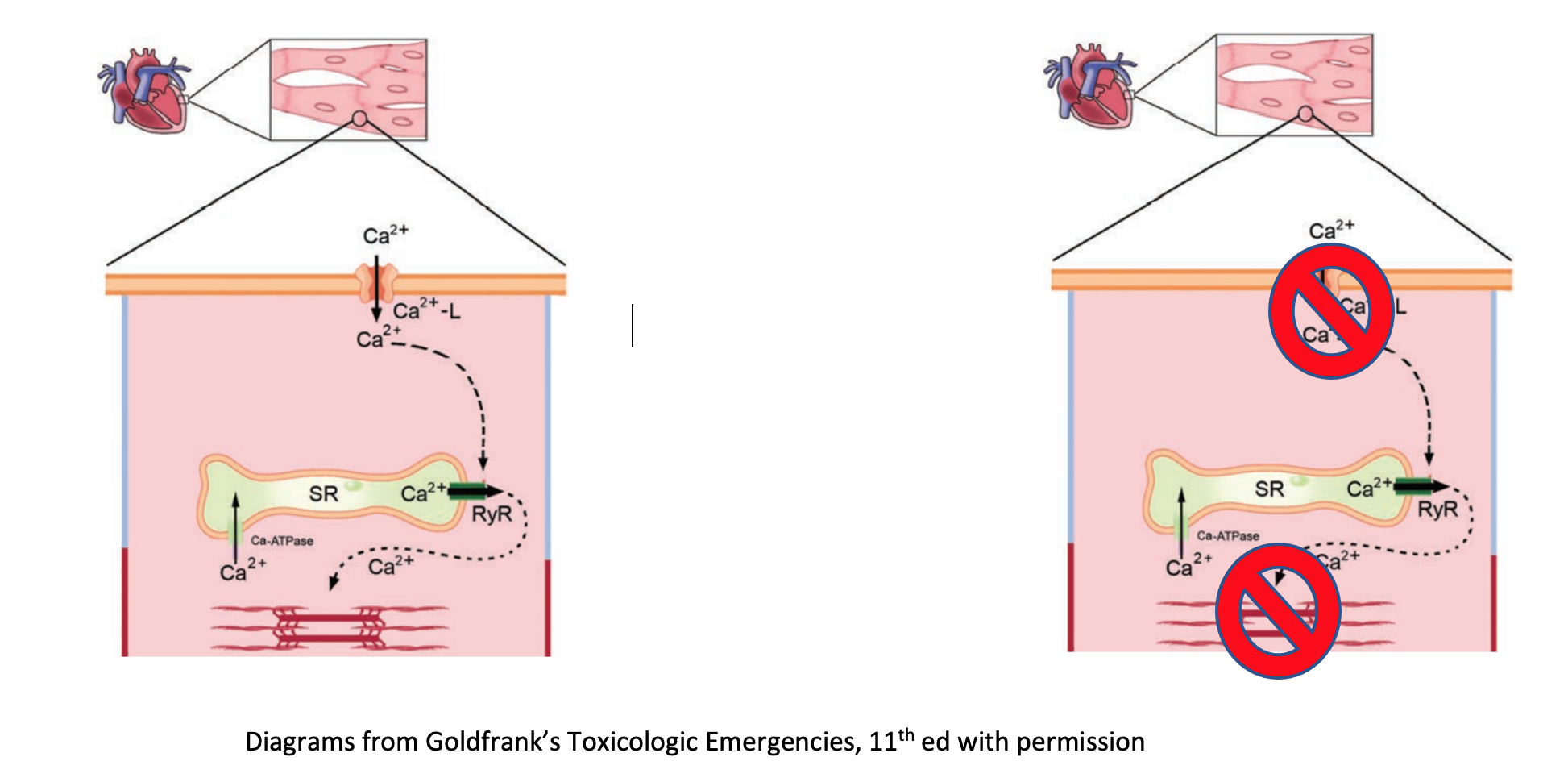
Mechanism of toxicity – Calcium Channel Blockers (CCB)
- In a normal state, depolarization of the myocyte opens the L-type calcium channel; resulting influx of calcium causes the concentration dependent release of more calcium from the sarcoplasmic reticulum, ultimately leading to myocyte contraction.
- Calcium channel receptors are also present on smooth muscle in vasculature and in pancreatic beta cells.
- Blockade at the trans-membrane receptor inhibits downstream calcium release. As a result, myocytes cannot contract, vasculature cannot contract, and beta cells cannot release insulin.
- Consequently results in hypotension, bradycardia, and hyperglycemia
Mechanism of toxicity – Beta Blockers
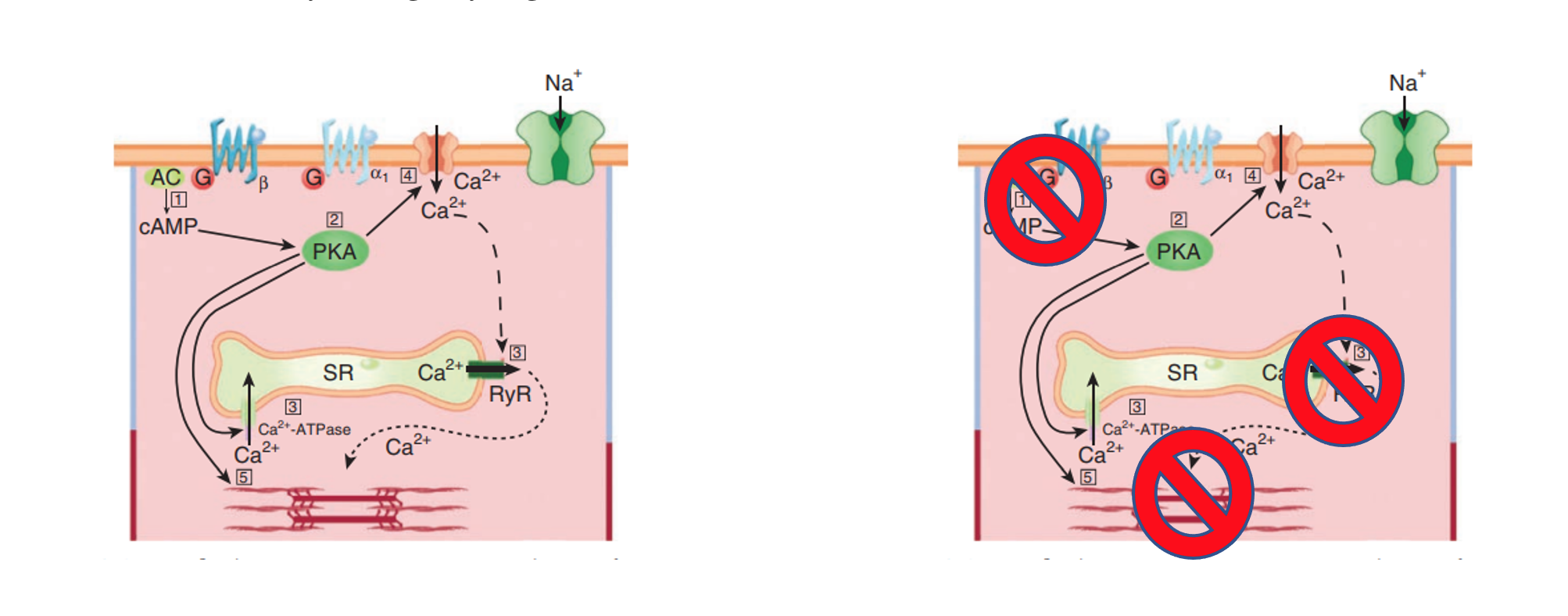
- Beta blockers block the beta-adrenergic G-protein coupled receptors on the heart, with different members of the class exerting variable effects on the other adrenergic receptors.
- Beta-adrenergic G-protein stimulation activates adenyl cyclase, increases cAMP, leading to activation of protein-kinase-A and other cAMP dependent protein-kinases. They result in the stimulation of pace-maker cells via effects of the calcium clock and increased myocyte contractility via effects on calcium channel influx and calcium storage in the sarcoplasmic reticulum.
- Beta blocker overdose produces profound bradycardia and hypotension due to blockade of these receptors.
- Can see hypoglycemia, useful for distinguishing between CCB/BB overdose
- At toxic doses, there also appears to be a catecholamine-independent effect on myocyte contractility through dysregulation of intracellular calcium release.
Overdose – general management:
- Consider GI decontamination if overdose is recent
- Activated charcoal 1g/kg up to 50g by mouth if patient is alert or by NG tube if airway has been secured
- Avoid in altered mental status as aspiration can lead to severe pneumonitis
- Gastric lavage/ whole bowel irrigation (WBI) can be indicated for recent massive overdose or extended-release formulations; however decision to proceed should be made in consultation with toxicology
- Activated charcoal 1g/kg up to 50g by mouth if patient is alert or by NG tube if airway has been secured
- Trial of calcium gluconate IV and glucagon IV
- If non-responsive to first line therapies and signs of cardiac depression present (hypotension, bradycardia, acidosis, elevated lactate, reduced EF on echo) escalate to high dose insulin euglycemic therapy.
- However, given the logistics of implementing HIET and the delayed onset of action, vasopressors should be initiated early to maintain adequate blood pressure until HIET becomes effective.
- Backup plans for mechanical supports such as venous-arterial ECMO should be included for patients refractory to HIET.
High Dose Insulin Euglycemic Therapy – why we do it:
- Mechanism not fully understood and is certainly multifactorial
- Insulin increases myocardial contractility via stimulation of phosphatidylinositol-3-kinase (PI3-K), resulting in reverse-mode sodium-calcium exchange and increased calcium concentration in the sacroplasmic reticulum (von Lewinski 2005).
- Stimulation of PI3K also increase myocardial contractility via calcium-independent mechanism (von Lewinski 2005).
- Stressed myocardium prefers glucose metabolism. Calcium channel blockers inhibits insulin release and decreases the number of cellular glucose transporters (GLUT); HIET improves glucose utilization.
- Other factors:
- Restores lactate metabolism in the heart (Kline 1997).
- Overcomes calcium channel blockade (Bechtel 2008).
- Largest evidence base is animal models:
- Multiple studies comparing HIET at doses 1-10 units/kg/hr to traditional vasopressors, glucagon, and placebo.
- Strong trend across different study designs for superiority of HIET.
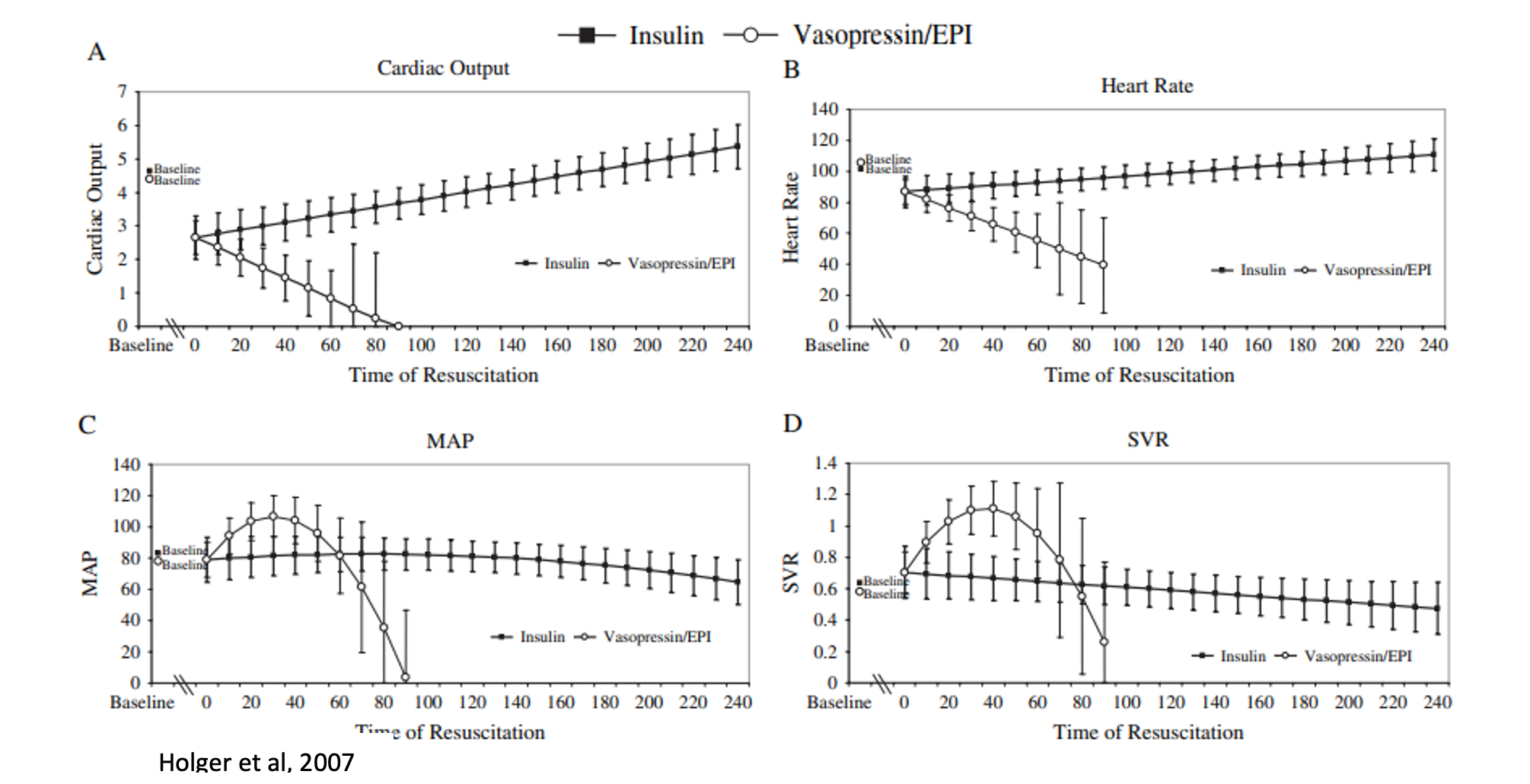
- HIET improves contractility without increasing SVR, while vasopressin and epinephrine transiently increase SVR/MAP but worsen cardiac output in anesthetized dogs given propranolol (Holger 2007).
HIET Implementation:
- Combination therapy of highly concentrated insulin and dextrose to maintain euglycemia.
- Potassium supplementation as needed
- Resource intensive requiring coordination across disciplines – emergency medicine, intensive care, toxicology, nursing, and pharmacy.
- Special attention should be directed at the insulin concentrations and programming of the infusion pump.
- Concentrated formulations of both dextrose and insulin to avoid iatrogenic fluid overload
- Insulin concentration for infusion should be at 10 units/mL.
- 70 kg patient, insulin at 3 units/kg/hr and dextrose at 0.5g/kg/hr
- Normal concentration of insulin 1 unit/mL and D10, would receive 560 mL of fluid/hr.
- HIET with insulin concentration of 10 units/mL and D50, would receive 90 mL of fluid/hr.
- Central line access required
- Takes about 30 minutes to take effect, can temporize with traditional vasopressors with the goal of turning them off when insulin is working.
- Insulin
- 1 unit/kg bolus
- 1 unit/kg/hr drip, titrated every 30-60 minutes to end organ perfusion to relative max of 10 units/kg/hr
- May not see change in MAP due to vasodilation from insulin; assess for improved cardiac output, urine output, acidosis, and lactate
- Some studies included max of 15 units/kg/hr without increased adverse effects (Page, 2018)
- Dextrose
- 0.5-1 g/kg/hr
- D50 or D20
- Central line access is required due to the high osmolarity of the glucose infusion.
- Supplement additional dextrose prn, if hypoglycemia occurs do not stop the insulin, increase the rate of the dextrose drip.
- Monitoring:
- Fingerstick glucose every 15 minutes for the first hour and after any up-titration; every 30 minutes if stable x 1 hour; then every hour for the duration of therapy.
- Will have prolonged need (18-24 hours) for dextrose supplementation after insulin is stopped.
- Potassium
- Shifted intracellularly by insulin, total body potassium is normal
- Replete to goal 3-3.5 mEq/L
- Goal is to prevent arrhythmia, not normalize potassium
- Monitoring:
- Every 1 hour while up or down-titrating insulin
- Every 4 hours while insulin requirement stable
Safety:
- Multiple retrospective reviews of HIET demonstrate high incidence of hypoglycemia and hypokalemia
- Page et al, 2018, 22 patients mixed CCB/BB overdose
- 16/22 hypoglycemia, 9/22 with severe (<45 mg/dL), none symptomatic
- 18/22 hypokalemia, none with arrhythmia
- Minimal clinical significance to these events, all responded promptly to additional supplementation.
- Reinforces importance of close monitoring and intervention.
Summary:
- High dose insulin with dextrose supplementation is indicated for patients with calcium channel blocker and beta blocker overdose and signs of cardiac toxicity.
- Mechanisms are not completely elucidated, but mostly related to the stimulation of PI3K.
- Evidence comes from animal models and 20 years of successful use in humans.
- Highly concentrated forms of insulin and dextrose via central line.
- Coordinate with toxicology, pharmacy, and nursing to avoid dosing errors.
- Takes 30 minutes to see effect, temporize with traditional vasopressors (norepinephrine, vasopressin).
- Titrate to end organ perfusion as MAP may not change significantly.
- Disposition to ICU.
- Back up plans for mechanical support such as venous-arterial ECMO such be considered early for refractory patients.
References:
Yuan TH, Kerns WP, Tomaszewski CA, et al. Insulin-glucose as adjunctive therapy for severe calcium channel antagonist poisoning. Journal of Toxicology: Clinical Toxicology. 1999;37(4):463-474.
Bechtel LK, Haverstick DM, Holstege CP. Verapamil toxicity dysregulates the phosphatidylinositol 3-kinase pathway. Academic Emergency Medicine. 2008;15(4):368-374.
von Lewinski D, Bruns S, Walther S, et al. Insulin Causes [Ca 2+ ] i -Dependent and [Ca 2+ ] i -Independent Positive Inotropic Effects in Failing Human Myocardium. Circulation. 2005;111(20):2588-2595.
Cole JB, Corcoran JN, Engebretsen KM, et al. Use of a porcine model to evaluate the risks and benefits of vasopressors in propranolol poisoning. J Med Toxicol. 2020;16(2):212-221.
Holger JS, Engebretsen KM, Fritzlar SJ, et al. Insulin versus vasopressin and epinephrine to treat β-blocker toxicity. Clinical Toxicology. 2007;45(4):396-401.
Kerns W, Schroeder D, Williams C, et al. Insulin improves survival in a canine model of acute β-blocker toxicity. Annals of Emergency Medicine. 1997;29(6):748-757.
Kline JA, Tomaszewski CA, Schroeder JD, et al. Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharmacol Exp Ther. 1993,267(2), 744-50.
Greene SL, Gawarammana I, Wood DM, et al. Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive Care Med. 2007;33(11):2019-2024.
Page CB, Ryan NM, Isbister GK. The safety of high-dose insulin euglycaemia therapy in toxin-induced cardiac toxicity. Clinical Toxicology. 2018;56(6):389-396.
St-Onge M, Anseeuw K, Cantrell FL, et al. Experts consensus recommendations for the management of calcium channel blocker poisoning in adults: Critical Care Medicine. 2017;45(3):e306-e315.
Goldfrank’s Toxicologic Emergencies. Edited by Nelson LS, Howland MA, Lewin NA, Goldfrank LR, Hoffman RS. McGraw-Hill Education, New York, 11th edition, 2019.
Hsu C, Wei J, Chen Y, Yang S, et al. Cellular mechanisms responsible for the inotropic action of insulin on failing human myocardium. The Journal of Heart and Lung Transplantation. 2006;25(9):1126-1134.
St-Onge M, Dubé PA, Gosselin S, et al. Treatment for calcium channel blocker poisoning: A systematic review. Clinical Toxicology. 2014;52(9):926-944.
Gummin DD, Mowry JB, Beuhler MC, et al. 2020 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th Annual Report. Clinical Toxicology. 2021; 59(12), 1282–1501.