Background
The new Surviving Sepsis Guidelines were released in January 2017 as an update to the 2012 guidelines. The 2012 sepsis criteria maintained the model of “early goal-directed therapy” (EGDT) as a guiding principle which became the standard of care after the groundbreaking Emmanuel Rivers’ study in 2001 (Rivers 2001). The 2017 Surviving Sepsis Guidelines now reflect the results of the PROCESS, PROMISE, and ARISE trials; 3 large multicenter studies demonstrating no significant difference in the primary outcome of mortality between EGDT and usual care. (ProCESS Investigators 2014, ARISE Investigators 2014, Mouncey 2015)
Definitions
- 2012 Guidelines
- Sepsis: A systemic manifestation of infection (i.e. Systemic Inflammatory response Syndrome [SIRS] criteria) + suspected infection
- Severe sepsis was defined as sepsis + end organ damage
- Septic shock was defined as severe sepsis + hypotension not reversed with fluid resuscitation (Dellinger 2012)
- 2017 Guidelines
- Redefine sepsis as agreed upon by The Society of Critical Care Medicine (SCCM) and the European Society of Intensive Care Medicine (ESICM) as the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) (Singer 2016)
- Sepsis:“life-threatening organ dysfunction caused by a dysregulated host response to infection.” End organ damage is identified as an acute change in total Sequential [Sepsis-related] Organ Failure Assessment score (SOFA) ≥2. (Rhodes 2017)
- Septic shock: A subset of sepsis “in which circulatory, cellular, and metabolic abnormalities are associated with a greater risk of mortality than with sepsis alone. These patients can be clinically identified by a vasopressor requirement to maintain a MAP ≥ 65mmHg and serum lactate >2mmol/L in the absence of hypovolemia” (Singer 2016)
- “Severe sepsis” category was deemed to be superfluous and is no longer recommended for clinical use
- SIRS criteria
- No longer considered in defining sepsis and septic shock
- Instead, adult patients outside of the ICU with suspected infection are identified as being at heightened risk of mortality if they have quickSOFA (qSOFA) score meeting ≥2 of the following criteria: respiratory rate of 22/min or greater, altered mentation, or systolic blood pressure of 100mmHg or less (Singer 2016)
Significant Changes
- Fluid Resuscitation
- Initial fluid resuscitation
- Unchanged from 2012 guidelines
- 30ml/kg of IV crystalloid fluid (normal saline or balanced salt solution) within the first 3 hours of sepsis presentation.
- Patients may require greater volumes of fluid as guided by frequent reassessment of volume responsiveness.
- Consider 4% albumin in refractory hypotension.
- Static fluid status measurements (i.e. Central Venous Pressure)
- No longer recommended as lone guiding principles as they carry limited value for measuring fluid responsiveness
- 2017 guidelines recommend the use of dynamic variables over static variables to predict fluid responsiveness (ie passive leg raise, pulse pressure variation, stroke volume variation)
- Weak suggestion to guide resuscitation to normal lactate
- Use clinical judgement. For instance, if patient has adequate BP and urine output and is down-titrating vasopressors, but has a persistently elevated lactate, additional fluid carries the risk of over-resuscitation.
- Initial fluid resuscitation
- Antibiotics
- First priority is source control and obtaining cultures. Cultures should be obtained prior to administration of antibiotics when feasible
- Give antibiotics within 1 hour of identification of septic shock
- Antibiotic Regimen
- Begin with broad spectrum coverage when the potential pathogen is not immediately obvious
- Narrow once pathogen identification and sensitivities are established
- Vancomycin
- Goal to achieve a trough of 15-20mg/L
- IV loading dose of 25-30mg/kg in septic shock
- For β-lactams, achieve higher Time-Dependent Killing (T>MIC) by increasing frequency of dosing
- Fluoroquinolones should be given at their optimal nontoxic dose
- Aminoglycosides should be dosed using once-daily dosing
- Average duration: 7-10 days is recommended in most patients
- Consider using procalcitonin to guide de-escalation of antibiotics
- Other:
- Vasopressors
- Useful in patients who remain hypotensive despite adequate fluid resuscitation
- Target mean arterial pressure (MAP) of 65mmHg
- First line vasopressor: norepinephrine
- Dose: start 2-12 mcg/min (no true maximum dose)
- Administer vasopressin (up to 0.03) and epinephrine as add-on therapies if not at target MAP or to decrease norepinephrine dose
- Consider inotropes in low cardiac output states i.e. septic cardiomyopathy, which can be common in these patients
- Steroids
- Indicated for patients with septic shock in which fluids and vasopressors fail to achieve hemodynamic stability
- Transfusion indicated in majority of patients only when hemoglobin <7.0g/dL
- Target glucose <180mg/dL
- Bicarb not recommended when pH>7.15
- Mechanical Ventilation (unchanged from 2012 guidelines)
- Lung Protective Ventilation Strategy
- Target a tidal volume of 6mL/kg of ideal body weight
- Plateau pressure of <30cm H20
- PEEP: incresae with FiO2 as per ARDSnet protocol
- Lung Protective Ventilation Strategy
- Vasopressors
- Recommend prone over supine position in patients with sepsis-induced ARDS and Pa/Fio2 ratio<150
- Recommendation against high frequency oscillatory ventilation/lung protective ventilation
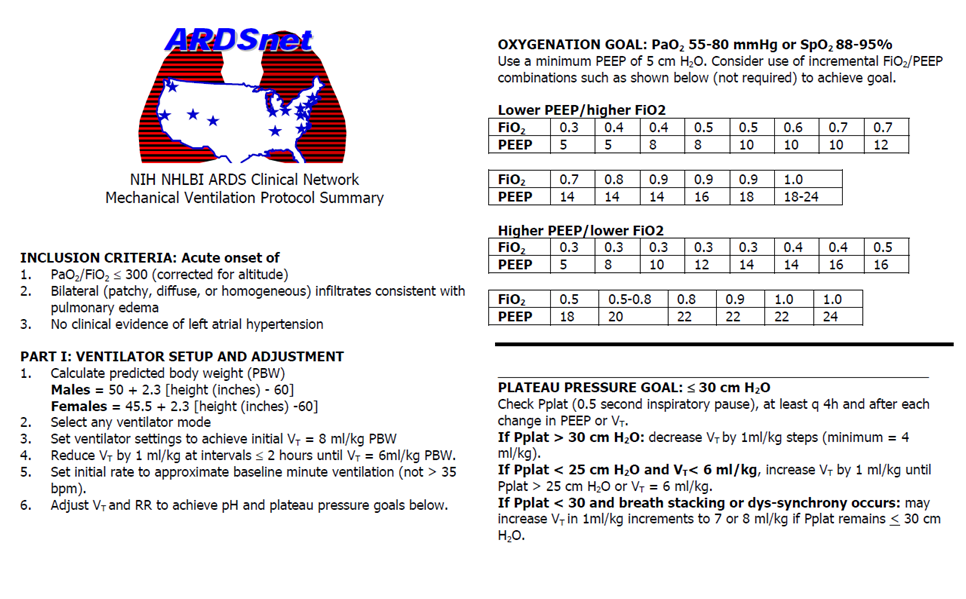
ARDSnet Protocol
Take Home Points
- Sepsis is defined as “life-threatening organ dysfunction caused by a dysregulated host response to infection”
- EGDT is no longer recommended for treatment of sepsis
- Begin broad spectrum, empiric antibiotic therapy within 1 hour in septic shock
- Initial fluid resuscitation of 30ml/kg, use frequent dynamic resuscitation markers, and the addition of vasopressors to target MAP of 65mmHg
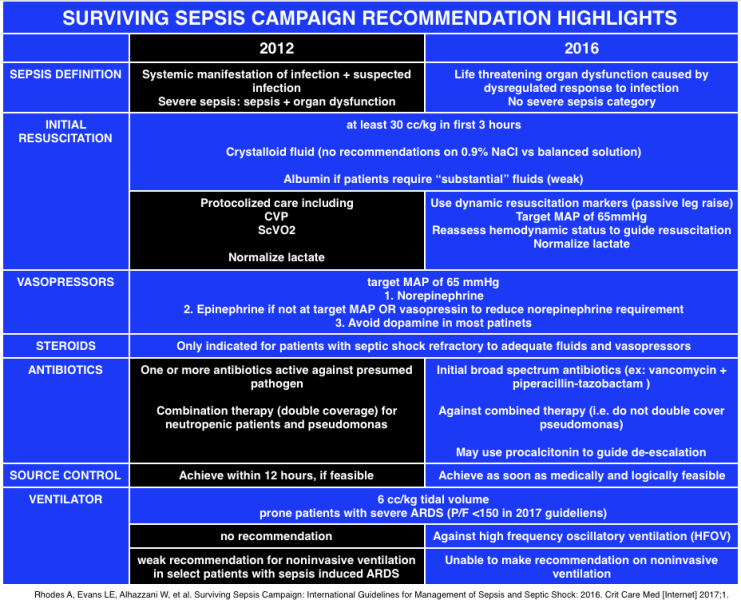
Surviving Sepsis Campaign Highlights (EMCrit)
Read More:
REBEL EM: Sepsis 3.0
REBEL EM: Fluid Responsiveness and the Six Guiding Principles of Fluid Resuscitation
FOAMcast: Surviving Sepsis Campaign Guidelines 2017
EMCrit: Surviving Sepsis Campaign (SSC) Guidelines 2016 Podcast
References
Rivers, E et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001; 345 (19): 1368-77. PMID: 11794169
ProCESS Investigators, Yealy DM, Kellum JA, Juang DT, et al. A randomized trial of protocol based care for early septic shock. N Engl J Med 2014; 370 (18): 1683-1693. PMID: 24635773
The ARISE Investigators, ANZICS Clinical Trials Group, Peake SL, et al. Goal-directed resuscitation for patients with early septic shock. N Engl J Med 2014; 371 (16): 1496-1506. PMID: 25272316
Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med 2015; 372 (14): 1301-1311. PMID: 25776532
Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 2013; 41 (2): 580–637. PMID: 23353941
Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315 (8): 801-10. PMID: 26903338
Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017; 45(3):486-552. PMID: 28098591

Thank you Brandon, Very helpful!
Hard to believe that so many people don’t know what is sepsis. Knowledge is power!
good
Good one,very helpful
Muy bueno