Background:
- Cardiogenic shock due to beta-blocker (BB) or calcium channel blocker (CCB) toxicity is frequent and potentially lethal.
- The most common cause of poison-induced cardiogenic shock is beta-blocker toxicity. In 2012 alone, there were 24,465 beta-blocker exposures (Mowry 2013).
- Calcium channel blocker overdose is less frequent than that of beta-blockers, but has been associated with the highest mortality rates among the cardiovascular drug overdoses(Woodward 2014).
Mechanism of Toxicity (Kerns 2011):
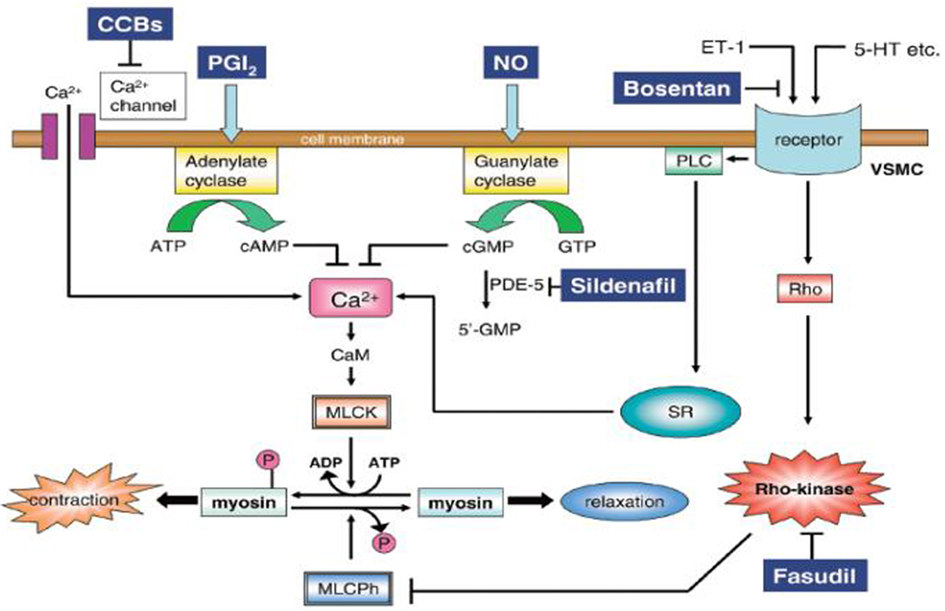
- BBs and CCBs lead to decreased intracellular calcium within the myocardial cells. This can lead to vasodilation, decreased systemic vascular resistance, bradycardia, conduction delay, decreased contractility, hypotension and cardiogenic shock.
- As the myocardium becomes stressed, it switches from catabolizing free fatty acids to catabolizing carbohydrates. The liver responds to this increased demand by releasing glucose via gluconeogenesis, ultimately resulting in hyperglycemia.
- Blockade of calcium channels leads to effects outside the cardiovascular system as well.
- CCB inhibits insulin secretion from the beta-islet cells of the pancreas. As a result of lower insulin levels, glucose cannot move into the myocardial cells at a rate sufficient to respond to demand.
- CCB inhibits lactate oxidation resulting in lactic acidosis
Traditional Management:
- Traditional management includes fluid resuscitation, atropine, cardiac pacing, calcium, glucagon and vasopressors. When these fail care may escalate to ECMO.
High Dose Insulin – How it Works:
- Under normal physiologic conditions the heart prefers to use free fatty acids as its primary energy source.
- In a stressed state the heart turns to prefer carbohydrate and insulin appears to facilitate this preference.
- In vitro and in vivo evidence has shown insulin’s positive inotropic and chronotropic effects(Reikeras 1985, Kline 1995).
- Even in a CCB poisoned animal model insulin increases myocardial glucose uptake resulting in improved contractility.
Using Hyperinsulinemia Euglycemia Therapy(Lugassy 2015)
- Hyperinsulinemia Euglycemia Therapy (HIET) Initiation:
- Intravenous bolus of regular insulin at a dose of 1 unit/kg.
- If serum glucose <250 mg/dL, concurrently administer a bolus of dextrose 25-50 g (or 0.5-1 g/kg) IV.
- HIET Continuous Infusion
- Regular insulin: start 0.5 – 1 unit/kg/hr
- Dextrose: 0.5 g/kg/hr (titrate to maintain glucose 110 – 150 mg/dL
- If the fluid overload is a concern, the insulin can be concentrated to 10 U/mL
- If hypoglycemia does occur, bolus with dextrose and/or increase dextrose infusion first before considering a decrease or cessation of insulin infusion.
- Continuous Monitoring
- Serum glucose every 30 minutes for 1-2 hours until stable
- Potassium every 1 hour
- Insulin bolus infusion can take 20-30 minutes to induce clinical inotropic/chronotropic effect.
- You may increase insulin infusion by 0.5-1 unit/kg/hr every 30-60 minutes (similar to administration of a pressor to maintain desired hemodynamic effect.)
- A wide range of continuous maintenance infusion of insulin for inotropic/chronotropic support have been reported with apparent safe use in the range of 3-10 Units/kg/hr.
- In addition to monitoring glucose and electrolyte levels, it may be prudent to monitor ejection fraction. Obtain a bedside echocardiogram upon arrival to estimate the patient’s ejection fraction. Repeat after 30-60 hours of insulin therapy. An improvement in EF is a good sign the therapy is working.
Adverse Effects:
- Most common adverse effects of HIET include hypoglycemia and electrolyte imbalances, especially hypokalemia. No irreversible adverse effects have been reported(Engebretsen 2011).
- In a case series of seven patients with severe calcium-channel blocker overdoses in which HIET was used, serum glucose and potassium levels were monitored closely (every thirty minutes until stabilized and then every 1-2 hours). One patient had a serum glucose concentration of <65 mg/dL that was rapidly corrected. Two patients had potassium concentrations <3.5 mEq/L, but neither had ECG signs of hypokalemia of arrhythmias. No patient had clinically significant hypoglycemia or hypokalemia(Greene 2007).
- Another case series examined twelve patients receiving HIET for drug-induced cardiogenic shock. Six patients developed a total of nineteen hypoglycemic effects and hypokalemia was seen in seven patients. No adverse arrhythmias were noted and no patients had adverse sequelae secondary to hypoglycemia or hypokalemia(Holger 2011).
- It is important to note that hypoglycemia may occur up to several hours after the insulin infusion has been completed.
Take Home Points
- HIET has been shown to be a safe and effective treatment for BB and CCB toxicity
- Although they have been rarely reported, hypoglycemia and hypokalemia are potential adverse events when using HIET. Monitor glucose and electrolytes closely while using this therapy.
References
Engebretsen KM et al High-dose insulin therapy in beta-blocker and calcium channel-blocker poisoning. Clin Toxicol 2011; 49(4): 277-283. PMID: 21563902
Greene SL et al. Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive Care Med 2007: 33(11): 2019-2024. PMID: 17622512
Holger JS et al. High-dose insulin: a consecutive case series in toxin-induced cardiogenic shock. Clin Toxicol 2011; 49(7): 653-658. PMID: 21819291
Kerns, W. Antidotes in Depth (A18): Insulin-Euglycemia Therapy. Goldfrank’s Toxicologic Emergencies 2015, 10th e. L. S. Nelson, N. A. Lewin, M. Howland et al. New York, NY, McGraw-Hill. Hyperlink
Kline JA et al. (1995). Beneficial myocardial metabolic effects of insulin during verapamil toxicity in the anesthetized canine. Crit Care Med 1995; 23(7): 1251-1263. PMID: 7600835
Lugassy DM et al. The Critically Ill Poisoned Patient. Emergency Department Resuscitation of the Critically Ill 2015. M. E. Winters, American College of Emergency Physicians.
Mowry JB et al. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol 2012; 51(10): 949-1229. PMID: 24359283
Reikeras O et al. Haemodynamic effects of high doses of insulin during acute left ventricular failure in dogs. Eur Heart J 1985; 6(5): 451-457. PMID: 3899650
Woodward C et al. High dose insulin therapy, an evidence based approach to beta blocker/calcium channel blocker toxicity. Daru 2014; 22(1): 36. PMID: 24713415

Great write up! As a hospital-based Critical Care/Flight Paramedic, I had the opportunity to be a part of the treatment team for a patient very recently that proved to be a perfect candidate for HIET. It was my first exposure to this particular method of treatment, and worked incredibly well. The patient did eventually require a captured airway and ventilatory support, but had an overall positive outcome.