Definition: A pulmonary embolism (PE) that results in hemodynamic compromise and end-organ hypoperfusion. The physical size of the PE does not differentiate a PE as massive or submassive but rather it is the patients physiologic response to the clot(s).
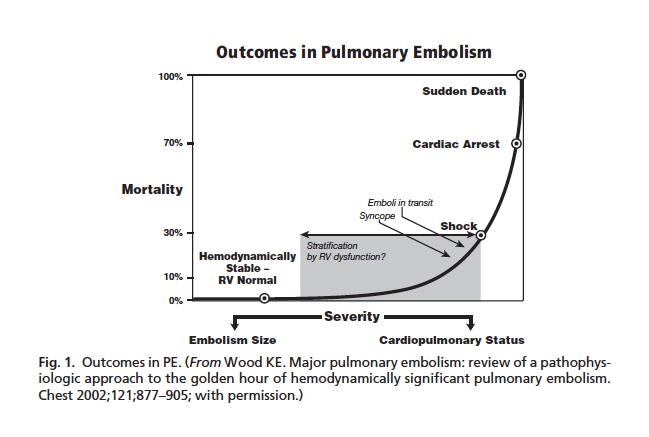
PE Mortality (Wood 2011)
Epidemiology: (Wood 2011)
- Incidence of PE: 600,000/year in the US
- Contributes to death in up to 200,000 patients
- Mortality
- Overall: 15-17%
- HD Stable: < 5%
- HD Unstable (Massive PE): 52-63%
- 2/3 of mortality occurs within 1st hour
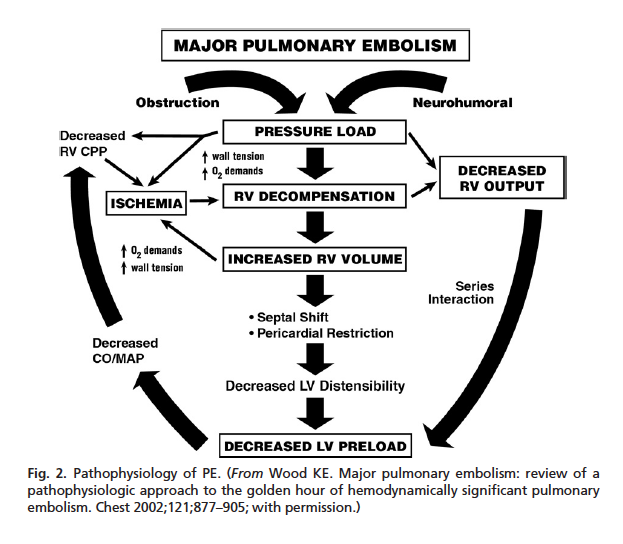
Severe PE Pathophysiology (Wood 2011)
Pathophysiology:
- Embolus in the pulmonary arteries causes mechanical obstruction to the right ventricle (RV) leading to RV strain
- Pulmonary embolus causes neurohormonal mediator release in the pulmonary vasculature contributing to increased pulmonary vascular resistance and increased RV strain
- Increasing RV strain leads to RV decompensation and failure resulting in decreased RV output
- The RV and left ventricle (LV) are in series. Failure of one ventricle leads to failure of the other.
- LV failure results in decreased cardiac output (CO), mean arterial pressure (MAP) and end-organ perfusion
- Decreased CO and MAP leads to worsening RV function (vicious cycle)
Presentation
- Signs
- Tachypnea
- Tachycardia
- Hypotension (may be transient)
- Hypoxia
- Increased jugular venous distension (if RV failure)
- Fever (typically low-grade)
- Lower extremity pain and swelling may be present if the patient has concomitant DVT
- Symptoms
- Shortness of breath/Dyspnea
- Chest pain
- Lightheadedness or Syncope
- Hemoptysis
- Leg pain (if concomitant DVT)
Differential Diagnosis
The differential diagnosis is broad and involves numerous diseases causing shock:
- ST-Elevation MI (STEMI)
- Cardiogenic Shock
- Tension Pneumothorax
- Cardiac tamponade
- Severe asthma or COPD exacerbations
- Anaphylactic Shock
- Septic Shock
- Hemorrhagic Shock (ruptured ectopic, occult trauma)
Basics: ABCs, IV, O2, Cardiac Monitor and 12-lead EKG
Airway, Breathing + Circulation
- Intubation may be necessary if the patient has significant hypoxia that does not respond to supplemental O2 or has respiratory fatigue
- This is likely to be a physiologically difficult airway
- Baseline hypoxia makes the risk of critical desaturation during RSI highly likely
- Hypotension may be worsened by induction medications and by institution of positive pressure ventilation
- Patients with shock are often acidemic and this acidosis may worsen during paralysis (lack of ventilation leads to rise in PaCO2)
- Maximize pre-intubation parameters
- Add high-flow nasal cannula to non-rebreather (NRB) face mask or non-invasive ventilation (NIV)
- Address hypotension
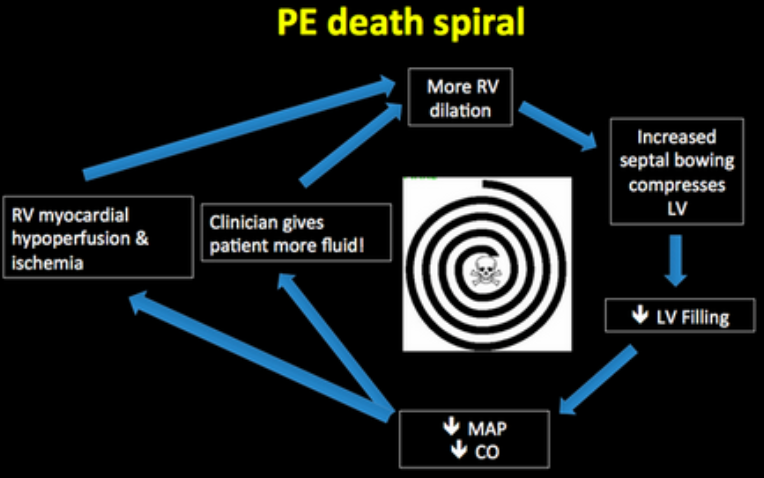
PE Death Spiral
- Cautious fluid administration
- Fluid boluses can lead to distension of the RV
- Distension of the RV can lead to compromise of the LV outflow tract leading to worsening hypotension
- Early use of vasopressors (norepinephrine/epinephrine)
- Consider using relatively “hemodynamically stable” induction medications (ketamine or etomidate) and reducing the dose of the induction agent
- Cautious fluid administration
- Minimize apneic time during which PaCO2 rises
Diagnostic Testing
- Patients with massive PE may not tolerate advanced diagnostic imaging (CT, V/Q scan) due to their hemodynamic instability
- Often, the diagnosis is made based on limited information from bedside assessment
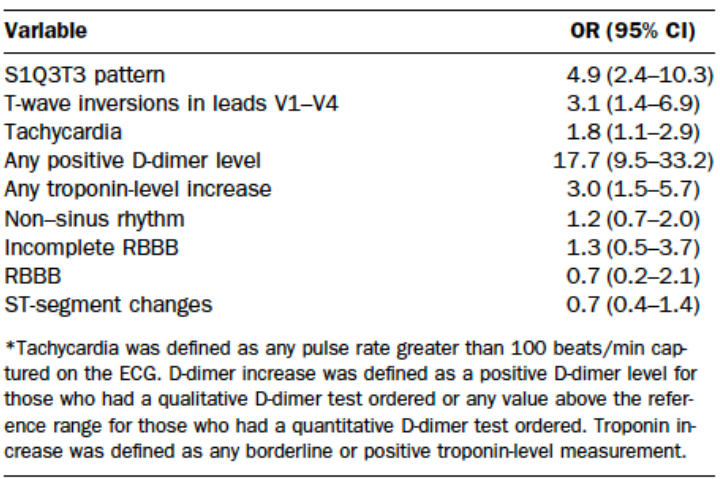
EKG Pulmonary HTN Findings (Marchick 2010)
EKG
- Overall, EKG has low sensitivity for PE. It is helpful in ruling out other causes of shock (i.e. STEMI)
- Findings of acute pulmonary hypertension frequently seen in acute pulmonary embolism (Marchick 2010)
- Simultaneous T-wave inversions in the anterior and inferior leads are uncommon but have a high specificity for PE (Witting 2012)
- ECG may demonstrate signs of myocardial ischemia, including ST elevations, which can mimic acute coronary syndrome (Shy 2015)
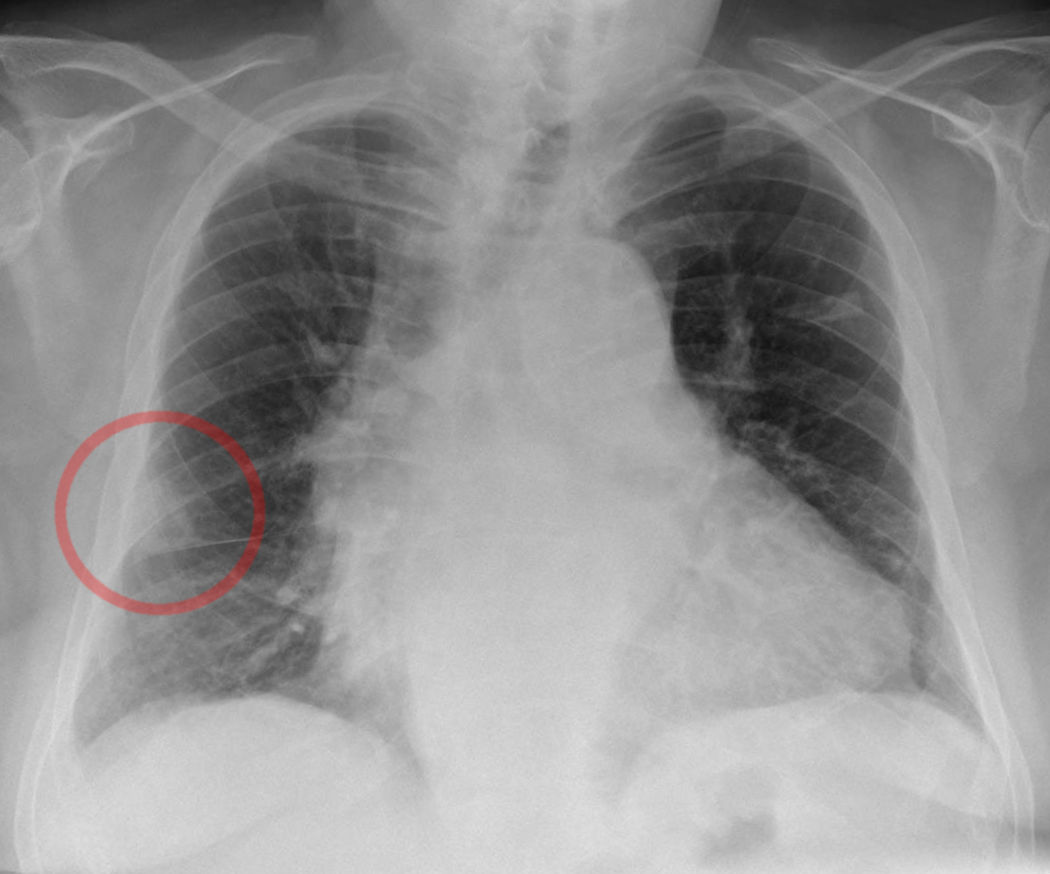
CXR: Hampton Hump
Chest X-ray
- Classic X-ray findings
- Hampton Hump: Dome-shaped, pleural based opacification secondary to pulmonary infarct. Pulmonary infarcts may also look similar to infiltrates (as seen in pnuemonia)
- Westermark Sign: Focal peripheral hyperlucency secondary to underperfusion of blood vessels (oligemia)
- Overall, low sensitivity and specificity for pulmonary embolism
- May be useful in finding or ruling out alternative diagnoses (i.e. pneumonia, pneumothorax)
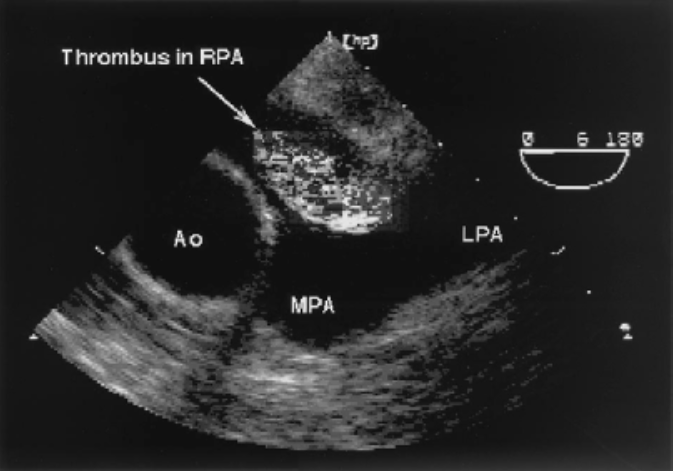
US Showing Clot in Transit (Goldhaber 2002)
Point of Care Ultrasound (POCUS) (Mookadam 2010)
- Allows for rapid elimination of alternative diagnoses
- FAST: Intra-abdominal Hemorrhage (occult abdominal trauma, ectopic pregnancy)
- Lung US: Pulmonary edema (in cardiogenic shock), pneumothorax
- Supporting Evidence for PE
- Cardiac US (Goldhaber 2002)
- Direct visualization of pulmonary embolism
- RV dilation and hypokinesis
- Pulmonary hypertension (Becattini 2010)
- Akinesia of the RV mid-free wall with normal motion of the apex (McConnell sign) (McConnell 1996)
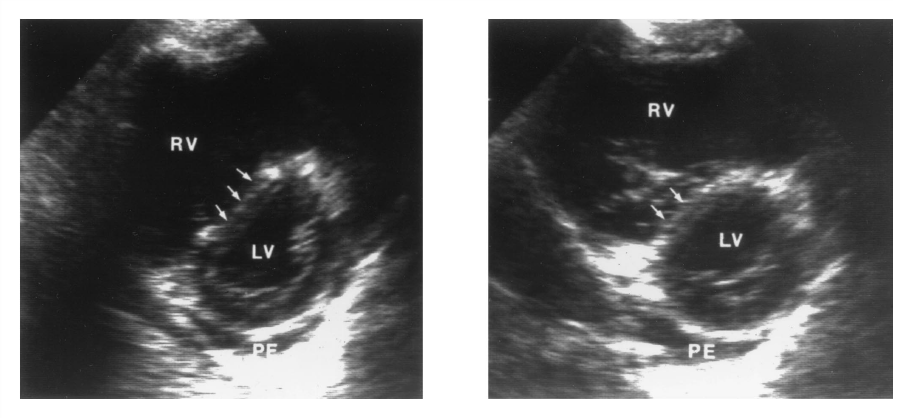
Interventricular Septal “Bowing”
- Original Study: Sensitivity 77%, Specificity 94%
- However, can see McConnell sign in RV infarction as well (Casazza 2005)
- Paradoxical interventricular septal bowing, i.e towards the left ventricle
- DVT study: presence of DVT in acute shortness of breath increases likelihood of PE as cause
- Cardiac US (Goldhaber 2002)
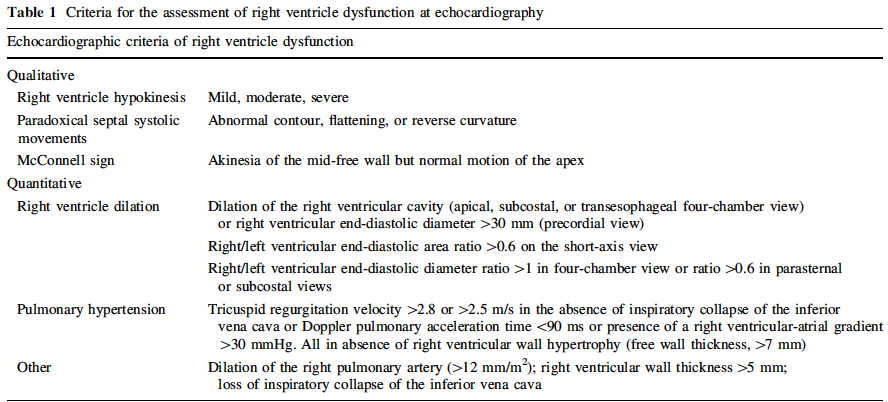
Signs of Right Ventricular Strain (Becattini 2010)
Directed Treatment
- Heparinization
- Prevents propagation of the PE(s) allowing the patients intrinsic fibrinolysis system to break down the clot
- Basis of treatment from small, prospective trial in 1960 (Barritt 1960)
- Patients with presumed PE based on symptoms + signs (advanced diagnostic imaging not available)
- Reduction in mortality from 5/19 to 2/16
- Included patients heterogenous in terms of disease severity (not all with signs of massive PE)
- Heparin options
- Unfractionated heparin
- Bolus: 80 units/kg IV
- Infusion: 18 units/kg/h IV
- Advantages: short half-life, can be partially reversed with protamine
- Low-molecular weight heparin (i.e. enoxaparin, dalteparin)
- Unfractionated heparin
- Systemic Thrombolytics
- Mechanism of action: binds to fibrin and converts tissue plasminogen to plasmin thus promoting fibrinolysis
- Systemic thrombolytics benefits + harms (Wan 2004)
- Death: 6.5% absolute reduction (6.2% vs. 12.7%) NNT = 16
- Recurrent PE: 3.2% absolute reduction
- Major bleeding: 10% absolute increase with thrombolytics (21.9% vs. 11.9%) NNH = 10
- Professional Organization Recommendation/Level of Evidence
- AHA: “Reasonable for patients with massive acute PE.” (Class IIa, Level of Evidence B) (Jaff 2011)
- Chest: “In patients with acute PE associated with hypotension who do not have a high bleeding risk, we suggest systemically administered thrombolytic therapy over no such therapy (Grade 2C) (Kearon 2012)
- ACEP: “Administer thrombolytic therapy in hemodynamically unstable patients with confirmed PE for whom the benefits of treatment outweigh the risks of life-threatening bleeding complications (Level B recommendation) (Fesmire 2011)
- Dose: Alteplase 100 mg IV over 2 hours (Kearon 2012). Can also consider giving bolus dose thrombolytics in patients with suspected or confirmed PE who have cardiac arrest.
- Hold heparin during infusion of alteplase
- Alternative Options
- Catheter directed thrombolytics (Piazza 2015)
- Intravascular catheter is placed into the pulmonary artery via either the femoral or internal jugular vein to the location of the clot
- Fibrinolytic therapy is delivered directly to the clot
- Allows for lower dose of fibrinolytic medication to be utilized and may reduce the risk of major bleeding and intracranial hemorrhage
- Surgical thrombectomy
- Catheter directed thrombolytics (Piazza 2015)
Take Home Points
- Massive PE is defined as any PE that results in hemodynamic instability.
- Patients with massive PE will often be too unstable for advanced diagnostic imaging (i.e. CT scan) requiring bedside diagnosis with history, physical examination and POC US
- Systemic administration of a fibrinolytic drug is a potentially life-saving therapeutic intervention with an NNT for death of 16. However, it comes with the significant risk of intracranial hemorrhage and major bleeding (NNH = 10)
asd
References:
Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial. Lancet 1960; 1: 1309-12. PMID: 13797091
Becattini C et al. Right ventricular dysfunction in patients with pulmonary embolism. Intern Emerg Med 2010; 5: 453-5. PMID: 20665963
Casazza F et al. Regional right ventricular dysfunction in acute pulmonary embolism and right ventricular infarction. Eur J Echo 2005; 6: 11-4. PMID: 15664548
Dong BR et al. Thrombolytic therapy for pulmonary embolism (review). Coch Data Syst Rev 2009; 3: 1-53. PMID: 19588357
Fesmire FM et al. Critical issues in the evaluation and management of adult patients presenting to the emergency department with suspected pulmonary embolism. Ann of Emerg Med 2011; 57: 628-52. PMID: 21621092
Goldhaber SZ. Echocardiography in the management of pulmonary embolism. Ann Intern Med 2002; 136: 691-700. PMID: 11992305
Jaff MR et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the AHA. Circulation 2011; 123: 1788-1830. PMID: 21422387
Kearon C et al. Antithrombotic therapy for VTE disease. Chest 2012; 141: e419S-4s. PMID: 22315268
Marchick MR et al. 12-Lead ECG findings of pulmonary hypertension occur more frequently in emergency department patients with pulmonary embolism than in patients without pulmonary embolism. Ann Emerg Med 2010; 55; 331-335. PMID: 19766353
McConnell MV et al. Regional right ventricular dysfunction detected by echocardiography in acute pulmonary embolism. Am J Cardiol 1996; 78: 469-73. PMID: 8752195
Mookadam F et al. Critical appraisal on the utility of echocardiography in the management of acute pulmonary embolism. Cardiol Rev 2010; 18: 29-37. PMID: 20010336
Piazza G et al. A prospective, single-arm, multi center trial of ultrasound-facilitated, catheter-directed, low-dose fibrinolysis for acute massive and submissive pulmonary embolism – the SEATTLE II study. JACC 2015; 8(10): 1382-93. PMID: 26315743
Shy B et al. Bedside ultrasound to evaluate pulmonary embolism masquerading as ST elevation myocardial infarction (STEMI). J Emerge Med 2015; 49(5): 703-4. PMID: 26164559
Wan S et al. Thrombolysis compared with heparin for the initial treatment of pulmonary embolism: a meta-analysis of the randomized controlled trials. Circulation 2004; 110: 744-9.PMID: 15262836
Witting MD et al. Simultaneous T-wave inversions in anterior and inferior leads: an uncommon sign of pulmonary embolism. J Emerg Med 2012; 43(2): 228-35. PMID: 22142671
Wood KE. Major Pulmonary Embolism. Crit Care Clin 2011; 27: 885-906. PMID: 22082519

Thank you for this informative review of the diagnosis and management of hemodynamically unstable pulmonary embolism. I am a nurse working on my Masters in Nursing Education and our assignment is a patient presenting with symptoms of PE. I needed to better understand the specific difference between what constitutes stable vs. unstable and you helped me to do just that. I am sharing this valuable resource with my professor and fellow students.
Thanks Swami, can you please add something about fluids?
Lorenzo – there’s probably little role for fluids in these patients. Fluid loading of any kind will cause dilation of the RV leading to bowing of the septum into the LV. This causes decreased LV outflow worsening shock. I would lead with pressors and hold fluids unless there’s signs of significant volume loss (not sure why there would be, though).
The NNT of 16 for death seem amazing from Wan´s metaanalysis. But doesn´t the 95% confidence interval include the unit (0.2-1.1), which means the difference is not statistically significant?