Background
Children with knee monoarthritis from Lyme disease and septic arthritis can have similar presentations. The early disseminated stage of Lyme disease, when knee monoarthritis would typically present, occurs 3-5 weeks post tick bite and a history of tick bite may not be present. In addition, synovial fluid cell counts do not distinguish between septic and Lyme arthritis and bacterial cultures and Lyme disease serology may take several days to result. Despite the similarities in presentation, the treatment of septic arthritis and Lyme arthritis is different. Lyme arthritis can be safely treated with oral antibiotics, while septic arthritis requires operative joint washout and initial parenteral antibiotics.
A retrospective cohort of children with knee monoarthritis was evaluated at 2 pediatric centers in Lyme disease-endemic areas, with the goal of deriving and internally validating a clinical prediction rule to identify children at low risk for septic arthritis who may not require invasive diagnostic techniques, such as arthrocentesis and operative joint washout (Deanehan, Pediatrics, 2013, PubMed ID: 23420916). The author’s concluded that “children with ANC 10 x 10³ cells per mm³ and ESR 40 mm/hour are at low risk for septic arthritis…our septic arthritis prediction model had the same sensitivity and higher specificity than the published Kocher criteria and can be used to assist clinical decision-making for the care of children with knee monoarthritis in Lyme disease-endemic areas.” However, the authors acknowledged that large validation studies are needed before widespread implementation of this model.
Clinical Question
In children with knee monoarthritis in Lyme disease endemic areas, does an absolute neutrophil count (ANC) of 10,000 cells/mm³ or an erythrocyte sedimentation rate (ESR) of 40 mm/hour, accurately identify children with and without septic arthritis?
Design
Observational: Prospective Cohort
Primary Results
- n=543 (research sample collected), n=457 (all data available for analysis)
- Median Age: 7 years, IQR (4-11 years), Male: 65.9% (358/543)
- Antibiotic Pretreatment: 7.0% (38/543)
Type of Arthritis
- Septic: 2.4% (13/543)(Staph aureus (8), Kingella kingae (3), Strep pyogenes (1), Pasteurella (1))
- Lyme: 43.1% (234/543)(56.8% with both (+) IgG and IgM, 38.9% (+) IgG alone, 4.3% (+) IgM alone Inflammatory: 54.5% (296/543)
No patients with septic arthritis were misidentified by the rule. The rule stratified a population with a 2.6% risk of septic arthritis into a group with a 7.8% risk (3-fold increase) of septic arthritis if the rule was positive and 0% risk of septic arthritis (2.6-fold decrease) if the rule was negative.
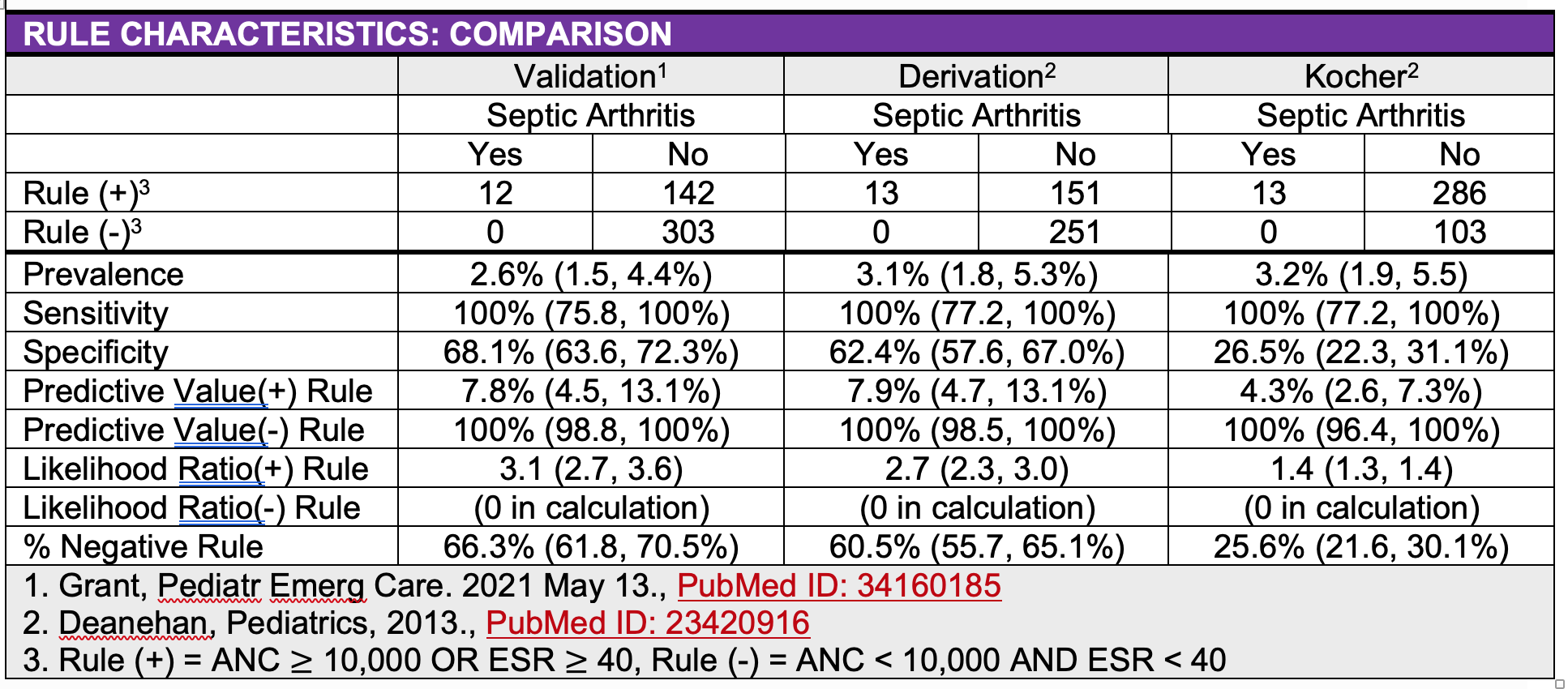
In this validation cohort, 66.3% (303/457) of patients had a negative rule and would be classified as low risk. In low risk patients, use of the rule could decrease the rate of arthrocentesis by 17.2%, operative joint washout by 5.3% and admission by 17.8%.
Strengths
- Multicenter (n=3), Broad validation to a Level II Clinical Decision Rule
- Rule consists of two objective laboratory criteria, easy to remember and apply
- Potential to reduce resource utilization
Limitations
- Convenience sample
- 3% of enrolled patients did not have both an ANC and ESR obtained
- Small sample size of patients with septic arthritis (n=13)
- Septic arthritis may have been underdiagnosed since not all children had synovial fluid cultures (27.1%). Though no patients were subsequently diagnosed with septic arthritis at follow-up.
- The proportion available for follow up was not presented
- Pretreatment with antibiotics (7.0%) before arthrocentesis may have caused false negative culture results.
- Did not assess newer acute phase reactants such as CRP and procalcitonin.
- The proportion of patients receiving unnecessary or incorrect antibiotics after enrollment was not presented in order to determine the potential impact of the rule on these outcomes.
- The predictive values would change in a non-Lyme endemic area
- Not applicable to arthritis at non-knee monoarthritis or polyarthritis
- Requires an impact analysis (Level I clinical decision rule) to confirm impact on resource utilization
Author's Conclusions
“We performed an external validation of the 2-factor septic knee arthritis clinical prediction rule in a multicenter prospective cohort of children undergoing evaluation for Lyme disease. This rule accurately identified children at low risk of septic arthritis. In Lyme disease–endemic areas, clinicians can use this tool to guide initial management for a child with knee monoarthritis to avoid potentially unnecessary and invasive procedures for low- risk children without missing a case of septic arthritis. Future studies are needed to evaluate the ability of newer biomarkers and novel diagnostics to more accurately identify children at the lowest risk for septic arthritis.”
Our Conclusions
The primary benefit of the rule is rule is reducing the rate of arthrocentesis, operative joint washout, admission and the use of unnecessary/incorrect antibiotics. The primary risk of using the rule is in missing septic arthritis. No patients with septic arthritis were misidentified by the rule. The lower limited of the 95% confidence interval for the predictive value of a negative rule was 98.8%, indicating that 1.2% (1 in 83) of patients with a negative rule could be misclassified as low risk for septic arthritis.
Potential Impact To Current Practice
The study’s results are applicable to pediatric patients with knee monoarthritis in Lyme endemic areas. The impact on resource utilization requires further study.
Read More
PEMCAR – Septic Arthritis Decision Rule Validation – PEC 2021