Background
The evaluation and management of febrile neonates remains controversial. Approximately, 10% of these patients will have a serious bacterial infection (SBI). Identification of the febrile neonate at low risk for serious bacterial infection could allow for a reduction in the rates of lumbar puncture, unnecessary antibiotics and hospital admission. The approach to these patients should evolve as the epidemiology changes and new diagnostic tests become available.
Clinical Question
In febrile neonates less than 60 days of age, do clinical and laboratory parameters adequately identify those at low risk for serious bacterial infection?
Design
Observational: Prospective cohort study (split derivation and validation sets)
Population
- Inclusion: Febrile (>38 C rectally) neonates less than 60 days of age who had a diagnostic evaluation for serious bacterial infection
- Exclusion: Critically ill, premature, preexisting medical conditions, indwelling devices and soft tissue infections
- Setting: 22 Pediatric Emergency departments in the PECARN network
Intervention (Rule Parameters)
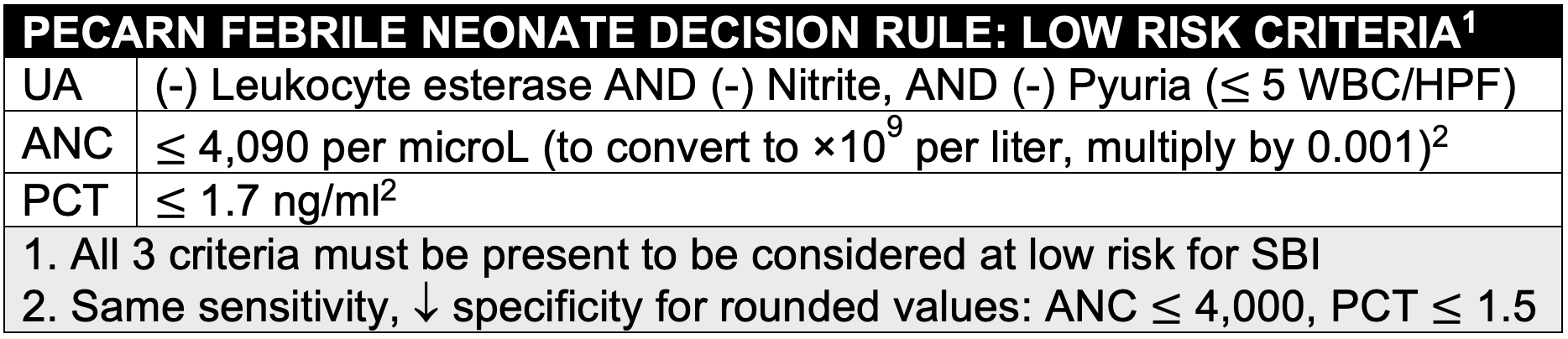
Evaluated predictors included: Age (≤28 days vs >28 days), temperature, duration of fever, Yale Observation Score, clinical suspicion of SBI, urinalysis, WBC count, absolute neutrophil count (ANC) and serum procalcitonin (PCT).
Control (Reference Standard)
Serious Bacterial Infection (SBI) was defined as one or more of the following:
- Bacterial Meningitis: Growth of a single pathogen in the CSF
- Bacteremia: Growth of a single pathogen in the blood
- UTI: Growth of single pathogen with:
- >1,000 CFU/ml (suprapubic aspiration) OR
- >50,000 CFU/ml (catheterization) OR
- 10,000-50,000 CFU/ml (catheterization) with an abnormal urinalysis ((+) leukocyte esterase OR (+) nitrites OR pyuria (> 5 WBC/HPF)
Outcomes
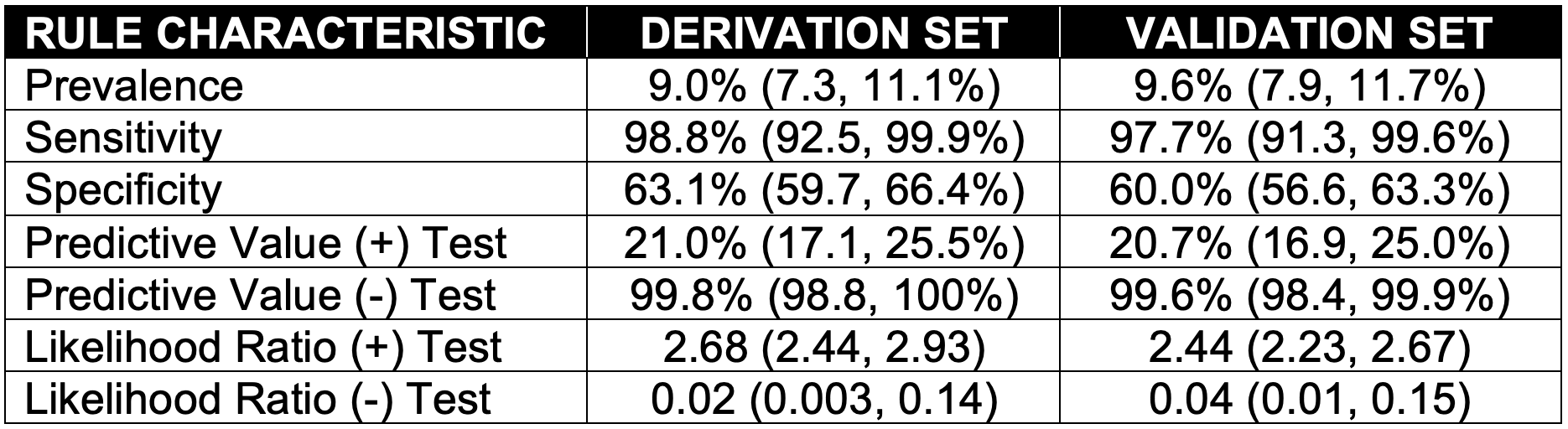
Rule Characteristics (SN, SP, Predictive values, Likelihood ratios)
Primary Results
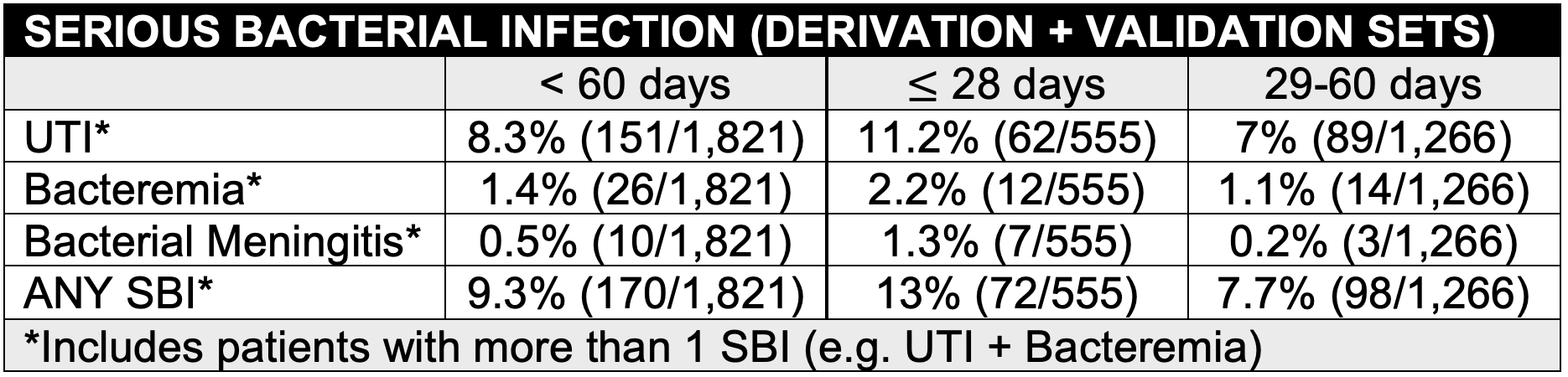
- <60 days: n = 1,821 (28 days: 30.5%, 29-60 days: 69.5%)
- UTI was the most common SBI and was present in 38% of those with bacteremia and 10% of those with bacterial meningitis
- E Coli (74%) and Group B Strep (7%) were the most common pathogens
- Neonates 28 days were more likely to have an SBI than those 29-60 days
- 1 patient (0.2%, 95% CI (0, 1.1%)) with bacteremia was missed in the derivation set. 2 patients (0.4%, 95% CI (0.1, 1.6%) with a UTI were missed in the validation set
- 76% in the study underwent an LP. The LP rate could be reduced by 20% if low risk infants did not have an LP. Antibiotic use and hospital admission could potentially be reduced by the same extent
Criteria and Results Tables presented in the “Read More” section
Strengths
- Multicenter: 22 pediatric EDs is the PECARN network. Results likely generalizable to patients in the ED setting meeting inclusion/exclusion criteria
- Large sample size of 1,821 febrile neonates (170 with SBIs)
- 3%, 95% CI (8.1, 10.8%) rate of SBI is similar to that commonly reported in the literature
- Statistically derived laboratory value cutoffs using recursive partitioning
- Similar rule characteristics in both the derivation and validation sets
- Low risk criteria include 3 objective laboratory parameters (UA, ANC, PCT)
Limitations
- Convenience sample: Potential for selection bias
- 24% of patients did not have a CSF culture: Potential for verification
- Viral testing not included in the analysis as was not available at all institutions in a timely manner to aid clinical decision making
- Only 30 patients had bacteremia and/or meningitis limiting conclusions that can be made about these outcomes individually
- Procalcitonin not consistently available in a time frame to aid clinical decision making in all EDs
- Level IV clinical decision rule that requires further validation before it can be applied clinically
Author's Conclusions
“We derived and validated an accurate prediction rule to identify febrile infants 60 days and younger at low risk for SBIs using 3 easily obtainable, objective variables: the urinalysis, the ANC, and serum procalcitonin. Once further validated, implementation of the rule has the potential to substantially decrease the use of lumbar punctures, broad-spectrum antibiotics, and hospitalization for many febrile infants 60 days and younger.”
Our Conclusions
The PECARN febrile neonate decision rule accurately identified infants with a low risk of serious bacterial infections. Few low-risk febrile neonates had a serious bacterial infection.
Potential Impact To Current Practice
If validated, use of the rule could potentially decreased the rates of lumbar puncture, unnecessary antibiotics and hospital admission in the febrile neonate. This must be balanced with the potential for missing febrile neonates with a serious bacterial infection. 1 patient (0.2%, 95% CI (0, 1.1%)) with bacteremia was missed in the derivation set. 2 patients (0.4%, 95% CI (0.1, 1.6%) with a UTI were missed in the validation set.
Bottom Line
Awaiting the broad validation of this rule and a procalcitonin level that is available in a timeframe to aid clinical decision making.
Read More
Study Criteria & Results Tables: