Background
Diverticulitis is a common cause of abdominal pain in the Emergency Department (ED) setting, especially in older patients. It has been reported as one of the 5 GI diseases most burdensome to the healthcare system at large in the United States. Diverticulitis is defined as inflammation of one or more diverticuli; outpouchings of the large intestine caused by herniation of the bowel mucosa into the colon wall. 85% of patients admitted with diverticular disease have “uncomplicated diverticulitis” (that is, without abscess, perforation, or other complications) and are treated conservatively with medical therapy (antibiotics). There has been a recent shift towards less aggressive care for this condition1.
Some have questioned the role of antibiotics at all in uncomplicated, mild acute diverticulitis. Prior this trial, two restrospective studies and a single small RCT have examined an observational approach vs empiric antibiotics for diverticulitis. These trials all suggested that antibiotics may be of no benefit in this scenario. Most providers continue to use antibiotics in the management of acute uncomplicated diverticulitis, and most guidelines would still suggest this approach.
As there are drawbacks to antibiotic therapy, including cost, adverse effects, allergies and resistance, limiting antibiotics use where it is unnecessary would be beneficial to patients.
Clinical Question
Does managing acute uncomplicated diverticulitis without antibiotics lead to a longer time to recovery, or an increased admission rate or occurrence of complications, compared to an antibiotic management strategy?
Population
Any patient with a first episode of left-sided, uncomplicated acute diverticulitis, confirmed by CT scan. Only “mild” cases were included (using scoring systems – Hinchey states 1a-b, and Ambrosetti’s mild stage).
Intervention
Observation either as an outpatient (if tolerating normal diet, temperature < 38oC, pain score < 4 with acetaminophen, capable of self-support prior to illness and patient amenable.
Control
Standard antibiotics care for acute diverticulitis without complication. Patients were admitted in the control arm for IV antibiotics. In keeping with local guidelines in the Netherlands, where the study was performed, amoxicillin-clavulanic acid was chosen; the regimen was a 10 day course, 1200mg QID, given IV for at least 48 hours, then switched to oral administration of 625mg TID if patient could tolerate.
Patients with an allergy received combo therapy with Ciprofloxacin and Flagyl.
Outcomes
Primary:Secondary: Days spent outside hospital in a 6-month period, readmission rate, occurrence of complicated diverticulitis (perforation, abscess, obstruction, fistula, severe bleeding), recurrence or continued diverticulitis, need for surgical intervention within 6 or 12 months, side effects of antibiotics in the control arm, and all cause mortality.
Design
Multicenter, open-label, randomized controlled trial. Outcome assessors were blinded to the group assignment.
Excluded
Previous radiologically proven diverticulitis, higher severity scores (Hinchey stages or Ambrosetii’s “severe” diverticulitis plus sepsis), or antibiotic use within the last 4 weeks.
Also excluded: radiologic findings suspicious for colonic malignancy, IBD, other highly morbid diseases (expected survival less than six months), contraindications to all antibiotics or renal failure, pregnant or breastfeeding patients, immunocompromised patients, unable to read questionnaire.
Primary Results
- Study Enrollment
- 893 patients assessed
- 570 were randomized to the allocation groups – 283 to observation and 287 to antibiotics.
- An additional 20 and 19 patients were excluded later for various reasons from each arm respectively
- 262 patients were analyzed for observation allocation, and 266 for antibiotics
- Follow up: 515/531 = 97%
- The two groups were similar in baseline demographic and prognostic factors, as laid out in Table 2.
Critical Findings
- Primary Outcome (Time to recovery)
- Median Time to Recovery (Primary Outcome)
- Observation group: 14 days
- Antibiotics group: 12 days
- Hazard Ratio: 0.91 (lower limit 0.78)
- Not statistically significantly different
- Within six months, 89.3% of patients in the observation group had full recovery, vs. 93.2% in the antibiotics group.
- Median Time to Recovery (Primary Outcome)
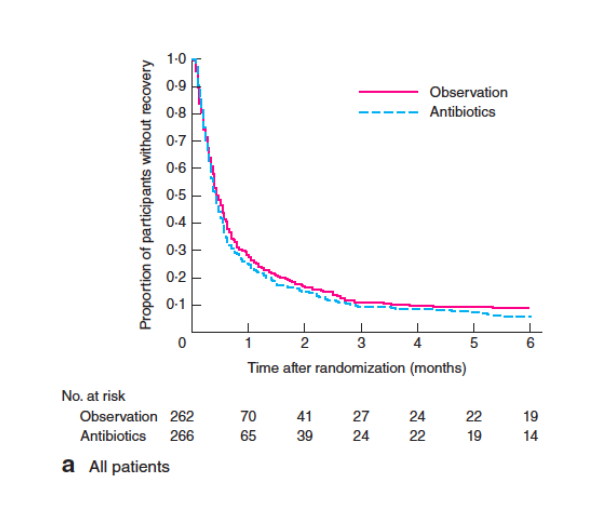
- Secondary Outcomes:
- None of the secondary outcomes had statistically significant differences
- Initial hospital discharge
- Observation group: 13%
- Antibiotics group: 0.4%
- Readmission rates
- Observation group: 17.6%
- Antibiotics group: 12%
- Complication rates
- Observation group: 3.8%
- Antibiotics group: 2.6%
- Recurrence rates
- Observation group: 3.4%
- Antibiotics group: 3.0%
Strengths
- Relatively large, multi-center trial
- Randomization appropriately performed
- Study asked a clear clinical question that was patient centered
- Follow up was excellent (97%) and similar in both groups
- Also attempted to track several secondary outcomes of importance, although not powered well for those outcomes.
Limitations
- While the primary outcome, time to recovery, was similar between the groups, a number of the the secondary outcomes were worse in the observation group. The study is not powered to give actionable information on secondary outcomes but this will need further investigation.
- Power analysis was performed post-hoc. Optimally, it should be performed prior to initiation of data collection
- The study was open-label (patients and doctors knew what treatment was administered). This likely biases the results towards the antibiotic arm
- The authors note that different centers had largely different accrual rates – which may introduce a selection bias
- A large amount of exclusion criteria (fever, sepsis, many common comorbidities) lead to a limitation in the population to which these results can be applied
- This trial was performed in the Netherlands. Regional dietary and genetic differences in a disease like diverticulitis (which is known to have different patterns and presentations in different regions of the world) limit the applicability of the findings for patients in North America.
- All patients had to have uncomplicated diverticulitis diagnosed on CT scan. Diverticulitis is often diagnosed without imaging.
Other Issues
- There may be difficulty in altering the approach to a disease in which other services, such as GI, will be heavily involved and see the patient for followup. They may not be open to such an approach.
- There are differences in availability of subsequent follow up (i.e. less able to guarantee follow up in an inner city population with lower health care access) that may make implementation less feasible.
Author's Conclusions
“Observational treatment without antibiotics did not prolong recovery and can be considered
appropriate in patients with uncomplicated diverticulitis.”
Our Conclusions
This large, multi-center RCT provides additional evidence that antibiotic therapy may not be necessary or helpful in the treatment of acute, uncomplicated diverticulitis. However, relevant secondary outcomes, including need for surgery or readmission, were found to favor the antibiotic group (though not statistically significantly different). Further investigation and external validation of this approach is necessary.
Bottom Line
The sum of the available high-quality evidence supports a “no antibiotics” approach to the management of uncomplicated, first episode diverticulitis. However, a paradigm shift in management will require discussion with appropriate consulting and follow-up disciplines in order to enact a comprehensive approach. Additionally, further research with examination of different population groups and safety data should be pursued.
Read More
EM Lit of Note: Antibiotics for Diverticulitis, the End Must Be Near