Background
Diabetic ketoacidosis (DKA) is an endocrine emergency. The standard of care of treating DKA is fluid resuscitation, electrolyte management, and intravenous insulin infusion in the intensive care unit (ICU) setting for close glucose and electrolyte monitoring. Recent research aims at investigating the treatment of DKA with subcutaneous insulin in non-ICU settings.
Clinical Question
Are subcutaneous insulin analogs in mild-to-moderate DKA efficacious, safe, and cost-effective, thus allowing treatment in non-ICU settings?
Population
This study occurred in an urban academic hospital with over 90,000 annual visits. Of these, 177 were mild-to-moderate DKA patients, defined as evidence of DKA (hyperglycemia, ketosis, and an anion gap) without severe features (HCO3 < 10 or arterial pH < 7.0). Of this group, 78 patients were placed in the SQuID protocol (subcutaneous insulin in DKA), and 99 were in the traditional cohort.
Intervention
SQuID protocol: IV fluids, electrolyte replacement, POC glucose every two hours, and insulin lispro (subcutaneous short-acting insulin). Patients were admitted to an inpatient observation unit managed by a hospital medicine service.
Control
A total of 99 mild-to-moderate DKA patients were placed in a traditional cohort during the study period.
Prior cases used as a control included 163 pre-intervention and 161 pre-COVID historical control patients.
Outcomes
Primary: operational impacts (EDLOS, ICU admission)
Secondary: fidelity, safety
Design
A prospective experimental study with retrospective data to evaluate outcome measures. Data was collected from August 1, 2021 – February 20, 2022. Providers screening a patient for DKA were given a Best Practice Advisory to consider placing the patient on SQuID protocol. Fidelity was examined by the frequency of required q2h glucose checks, safety by seeing how many patients required rescue dextrose for hypoglycemia, and operational impacts including ED LOS and ICU admission.
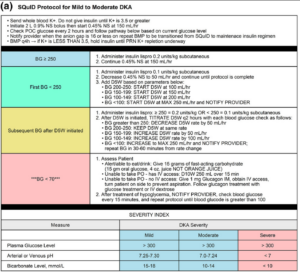
Image from cited article.
Excluded
- Patients with severe DKA (HCO3 < 10 mmol/L or arterial pH < 7.0)
- Patients <18 years of age
- Exclusion criteria for the SQuID protocol:
- Pregnancy
- Serious infections
- Concerns for myocardial infarction
- Altered mental status
- Active comorbidities (ESRD, CHF, on immunosuppressants)
- Need for a surgical intervention
- ED or inpatient team determined the patient was too ill for the designated floor (an inpatient observation unit run by hospitalist physicians)
Primary Results
177 patients with mild to moderate severity DKA (78 SQuID, 99 traditional)
-
- 76 were admitted to an ICU
- Among those admitted to a medical floor:
- 73 patients were managed on the SQuID protocol
- 28 were managed on an insulin infusion
Fidelity
- High fidelity for patients in the SQuID pathway
Safety
- No differences in safety issues in patients in the SQuID pathway compared to the traditional cohort
EDLOS
- Significantly shorter for patients in the SQuID pathway
ICU admission
- Reductions in ICU admissions were observed though not statistically significant
Strengths
Variety of controls, including pre-COVID, pre-protocol, and current controls.
Limitations
Generalizability (single center, level 1 urban hospital with limited ICU beds available).
Implementation requires a new hospital specific protocol involving provider education and inpatient unit for SQuID protocol.
Author's Conclusions
“In this single academic medical center study, subcutaneous fast-acting insulin analogs for the treatment of mild to moderate–severity diabetic ketoacidosis in the ED was effective, demonstrated equivalent safety, and reduced ED length of stay.”
Potential Impact To Current Practice
ED boarding continues to be a major nationwide issue, and ICU bed availability is often limited. Implementing a DKA protocol where patients’ safety and fidelity are not compromised and patients can be treated promptly is ideal for both patients and throughput for EDLOS.
Bottom Line
Using subcutaneous fast-acting insulin for diabetic ketoacidosis can be safe for patients with mild to moderate DKA. However, significant medical education and protocol implementations still need to be implemented to practice a protocol like SQuID safely.
