Background:
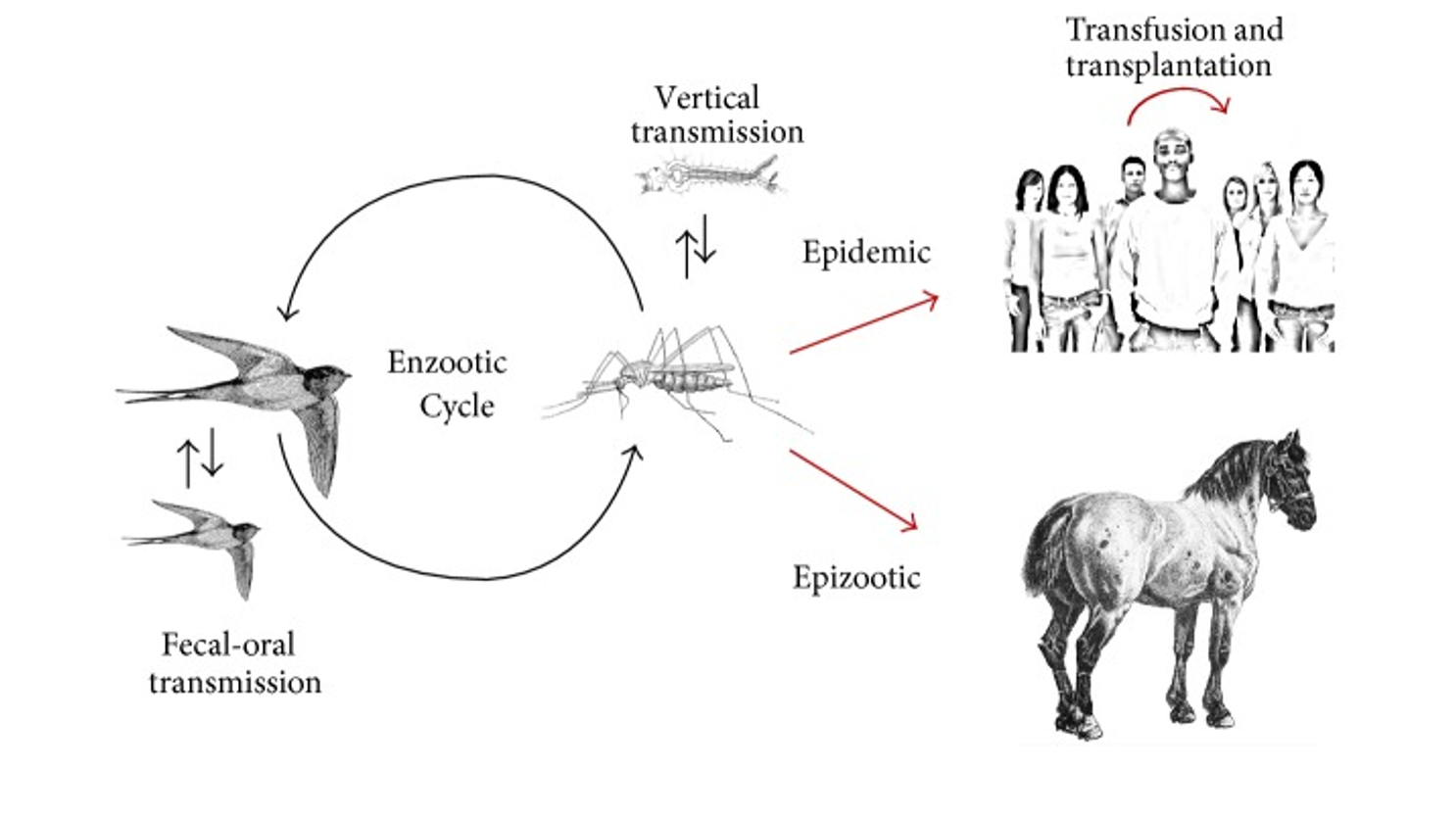
West Nile Virus (WNV) is a mosquito-borne flavivirus and neuropathogen that causes a febrile illness, encephalitis, meningitis and flaccid paralysis. The virus is indigenous to Africa, Asia, Europe, and Australia. WNV is maintained in nature in a mosquito-bird-mosquito transmission cycle primarily involving Culex mosquitoes. Clinical manifestations of WNV infection range from uncomplicated West Nile fever to fatal meningoencephalitis (although 80% of cases are asymptomatic). Incidence of severe neuroinvasive disease and death increases with age.
Epidemiology:
- First known to North America in 1999 when it caused an epidemic of meningoencephalitis in New York City. (Nash 2001)
- It is the leading cause of domestically acquired arboviral disease in the United States and is endemic in 48 US states and in all Canadian provinces. (Peterson 2019)
- Major outbreaks have also occurred in Romania, Italy, Greece, the Balkans, Ukraine, Russia, northern Africa and Israel. (Chancey 2015)
- From 1999 to 2018, 50,830 confirmed and probable cases of WNV disease and 24,657 cases of neuroinvasive disease, were reported to the CDC. (CDC 2019)
- Most human infections with WNV are due to mosquito bites. Though rare, transmission can also occur through blood transfusion, through organ transplantation and transplacentally.
- Incidence increases with warmer temperatures and peaks in the late summer and early fall when environmental conditions promote viral amplification, and increasing numbers of mosquitoes that bite humans and birds become infected. (Peterson 2019)
Pathophysiology:
- WNV is a single-stranded RNA virus of the family Flaviviridae, genus Flavivirus.
- The mosquito infects the host during feeding when virus-containing saliva is injected and inoculates subcutaneous tissue. Viral dissemination then occurs in three phases: replication in keratinocytes and dermal dendritic cells, dissemination to visceral organs through draining lymph nodes and viremia and lastly, invasion of the central nervous system.
- Of note, birds (especially crows and jays) sicken and die when infected; some locales offer postmortem evaluation for birds to assess for the presence of WNV in the environment. (Mostashari 2003)
- Among humans who become infected, approximately 20%-25% develop West Nile fever while 1 in 150 to 250 develop neuroinvasive disease. (Peterson 2013)
- The mechanisms by which WNV enters the CNS are not precisely known but is postulated to include crossing of blood-brain barrier through cytokine-mediated vascular permeability, a mechanism where infected tissue macrophages are trafficked across the blood-brain barrier or retrograde axonal transport of the virus to the central nervous system via infection of olfactory or peripheral neurons. (Samuel 2006)
- In the CNS, persistent viral replication occurs which may lead to long-term neurological sequelae.
- WNV–associated paralysis results from destruction of the anterior horn cells of the spinal cord.
- The incubation period for clinical illness generally ranges from 2 to 14 days, but prolonged incubation periods of up to 21 days can occur in immunocompromised patients.(CDC 2019)
Clinical Presentation:
- 80% of cases are asymptomatic while 20% develop symptoms.
- Most common symptoms — also known as West Nile Fever — develop on average 2-6 days post mosquito bite: fever, lymphadenopathy, GI symptoms (abdominal pain, vomiting, diarrhea), myalgias, malaise, headache, +/- morbilliform maculopapular rash.
- May also present with eye pain, pharyngitis, or orchitis; or symptoms that are consistent with rhabdomyolysis, myocarditis, or pancreatitis.
- WNV neuroinvasive infection (1 in 150-250): often overlap of encephalitis (fever, AMS, Parkinson-like tremors, sensory deficits) with aseptic meningitis (headache, photophobia, meningismus) and/ or myelitis (flaccid paralysis, polio-like features). May also present with mild confusion, lethargy, encephalopathy, coma.
- Symptoms that may persist after acute illness include fatigue, memory impairment, weakness, headache, and balance problems.
Diagnosis:
- WNV serum and CSF EIA IgM using enzyme-linked immunosorbent assay (MAC-ELISA); PCR is less sensitive but if positive it is still diagnostic. CSF IgM may turn positive as soon as day 3 after symptom onset and may persist for up to 90 days.
- Those recently vaccinated for yellow fever or Japanese encephalitis, or people recently infected with other flaviviruses such as St. Louis encephalitis or dengue may have positive results on IgM antibody tests for WNV.
- The plaque-reduction neutralization test can be used to distinguish serologic cross-reactions among flaviviruses.
- If the initial MAC-ELISA test is negative and WNV infection is still suspected, a repeat serum MAC-ELISA can be obtained ~10 days later.
- Nucleic acid amplification testing is useful in immunocompromised patients, those requiring an urgent diagnosis, and patients with previous flavivirus exposure whose serologic results may be ambiguous.
- Other findings include CSF with elevated protein (100-1000), pleocytosis with lymphocytic predominance, normal to low WBC, and negative glucose.
- In some patients T2 weighted brain MRI showed enhancement of the leptomeninges and periventricular areas.
ED Management:
- Treatment is primarily supportive.
- Studies demonstrating efficacy of specific agents are limited.
- Therapeutic agents that have been considered include IVIG, monoclonal antibodies, corticosteroids, and acyclovir.
- Public health measures focus on preventative strategies such as limiting the number of infected mosquitos using insecticides, encouraging use of insect repellants, and testing of donated blood.
Take Home Points:
- Most people infected with WNV are asymptomatic (80%). Manifestations of WNV infection range from fever to severe neuroinvasive disease. Severity of illness correlates with age and immunocompromised status.
- WNV illness should be considered in anyone with a febrile or acute neurologic illness who has had recent exposure to mosquitoes, blood transfusion, or organ transplantation, especially during August and September in endemic areas.
- If WNV infection is suspected, serum testing for WNV IgM antibodies should be performed using an enzyme-linked immunosorbent assay (MAC-ELISA). Patients with neurologic symptoms should have a lumbar puncture CSF should be tested for IgM antibodies.
- Treatment is largely supportive. Use of specific therapeutic agents have been described but evidence for efficacy for each one is lacking.
References:
Chancey C, Grinev A, Volkova E, Rios M. The global ecology and epidemiology of West Nile virus. Biomed Res Int. 2015;2015:376230. doi: 10.1155/2015/376230. Epub 2015 Mar 19. PMID: 25866777.
Mostashari F, Kulldorff M, Hartman JJ, et al. Dead Bird Clusters as an Early Warning System for West Nile Virus Activity. Emerging Infectious Diseases. 2003;9(6):641-646. doi:10.3201/eid0906.020794.
Nash, Denis, et al. “The outbreak of West Nile virus infection in the New York City area in 1999.” New England Journal of Medicine 344.24 (2001): 1807-1814. PMID: 11407341
Petersen LR. Epidemiology of West Nile Virus in the United States: Implications for Arbovirology and Public Health. J Med Entomol. 2019 Oct 28;56(6):1456-1462. doi: 10.1093/jme/tjz085. PMID: 31549728.
Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. JAMA. 2013 Jul 17;310(3):308-15. doi: 10.1001/jama.2013.8042. PMID: 23860989; PMCID: PMC4563989.
Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006 Oct;80(19):9349-60. doi: 10.1128/JVI.01122-06. PMID: 16973541; PMCID: PMC1617273.
West Nile Virus Cumulative Maps 1999-2019. United States: Center For Disease Control and Prevention, 2019. https://www.cdc.gov/westnile/statsmaps/cumMapsData.html