Background:
The lumbar puncture (LP or spinal tap) is a procedure that has been in the medical arsenal since first described in 1891 by German physician Heinrich Quincke. It can be both diagnostic and therapeutic, utilized in the diagnosis (meningitis, Guillain-Barre syndrome, subarachnoid hemorrhage) and symptomatic treatment (idiopathic intracranial hypertension, normal pressure hydrocephalus) of diseases of the central nervous system, respectively.
LP is an invasive procedure in which a spinal needle is inserted into an intervertebral space to obtain samples for cerebrospinal fluid testing. Prior to needle insertion, landmarks must be identified to ensure procedural success. LPs are one of the most challenging procedures for clinicians. A study by Duniec et al. reported that landmark-based assessment of intervertebral space can be inaccurate up to 30% of the time, in part due to patients’ varying body habitus and positional limitations. This, of course, is less than ideal, as the inability to correctly identify landmarks can lead to repeated attempts, patient discomfort, and increased rate of post-procedural complications.
Ultrasound can be a useful tool to ensure procedural success of a LP on the first attempt. It can optimize visualization of the intervertebral spaces and spinous processes to provide a better static approach for performance of the procedure.
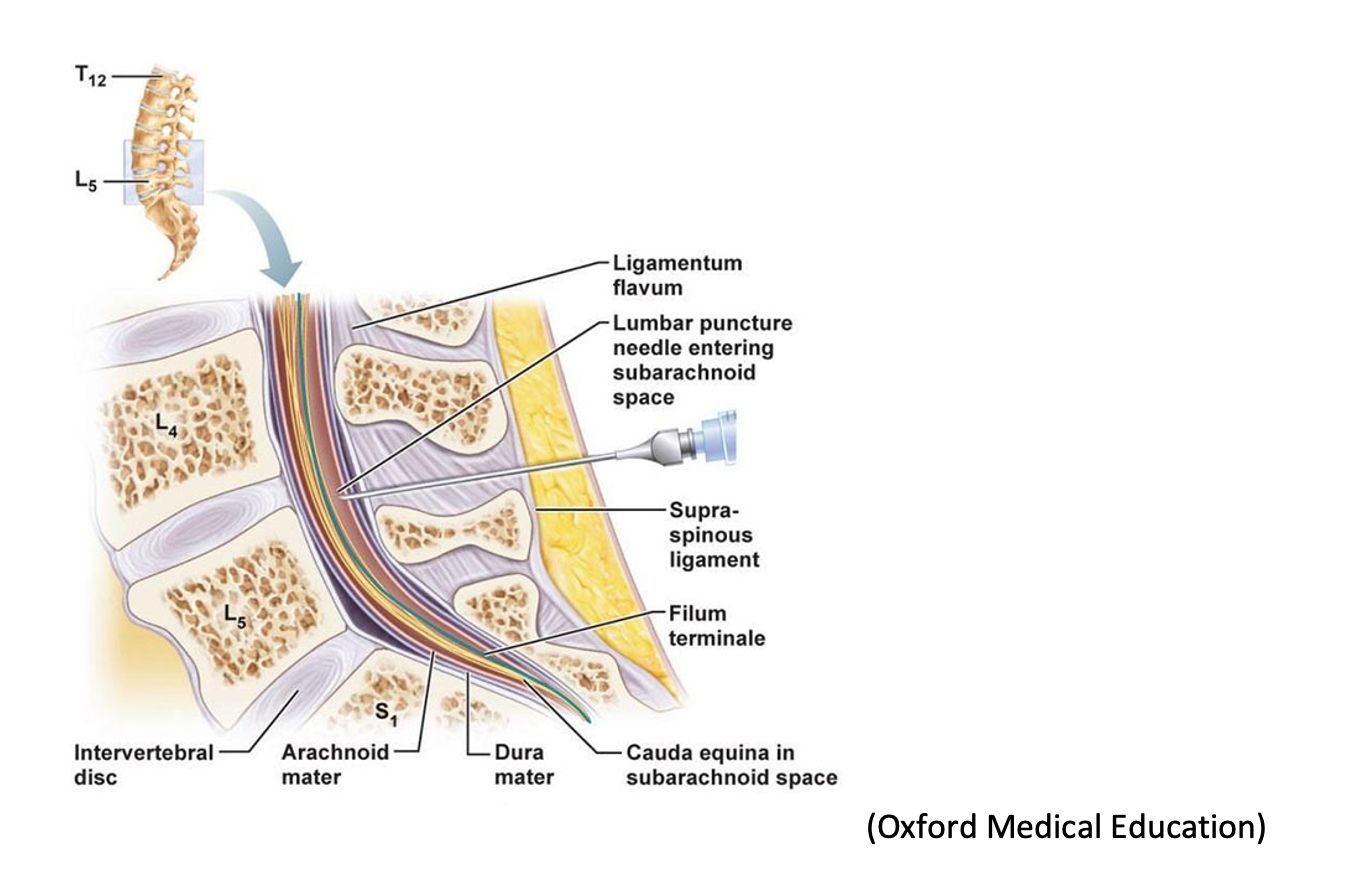
Technique:
Probe selection:
The linear probe is more ideal for pediatric patients and adults with a leaner body habitus. For all other patients, a curvilinear probe would be more preferable.
Positioning:
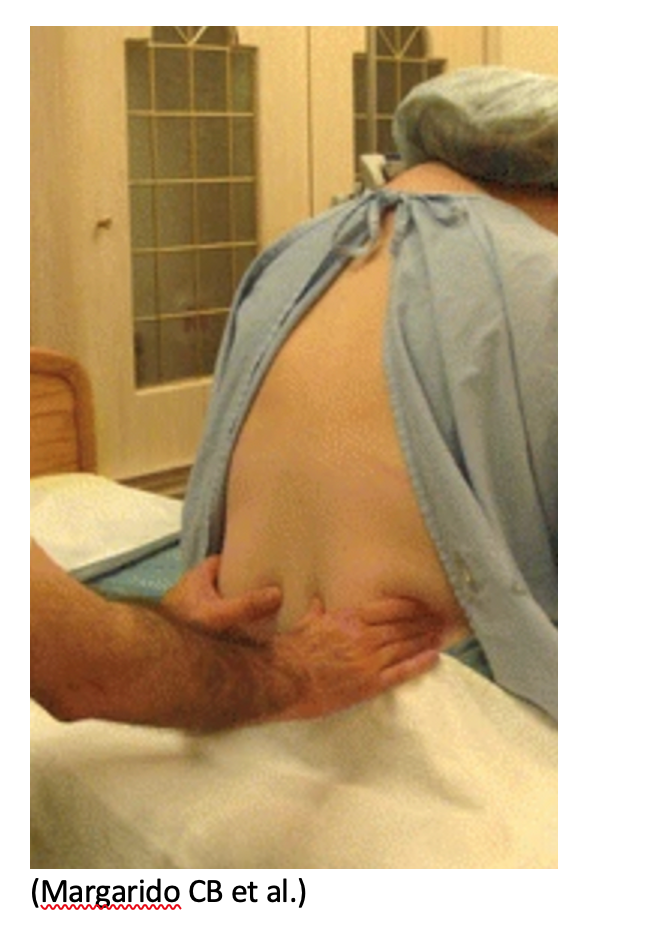
A LP can be performed in either the lateral recumbent/prone position or upright position. To accurately measure the patient’s intracranial pressure, the procedure should be performed in the lateral recumbent/prone position. However, you can outline the intervertebral spaces using the ultrasound in either position.
- Find Tuffier’s Line: Tuffier’s line is the transverse line connecting the superior aspects of the posterior iliac crests, passing through the L3-L4 intervertebral space.
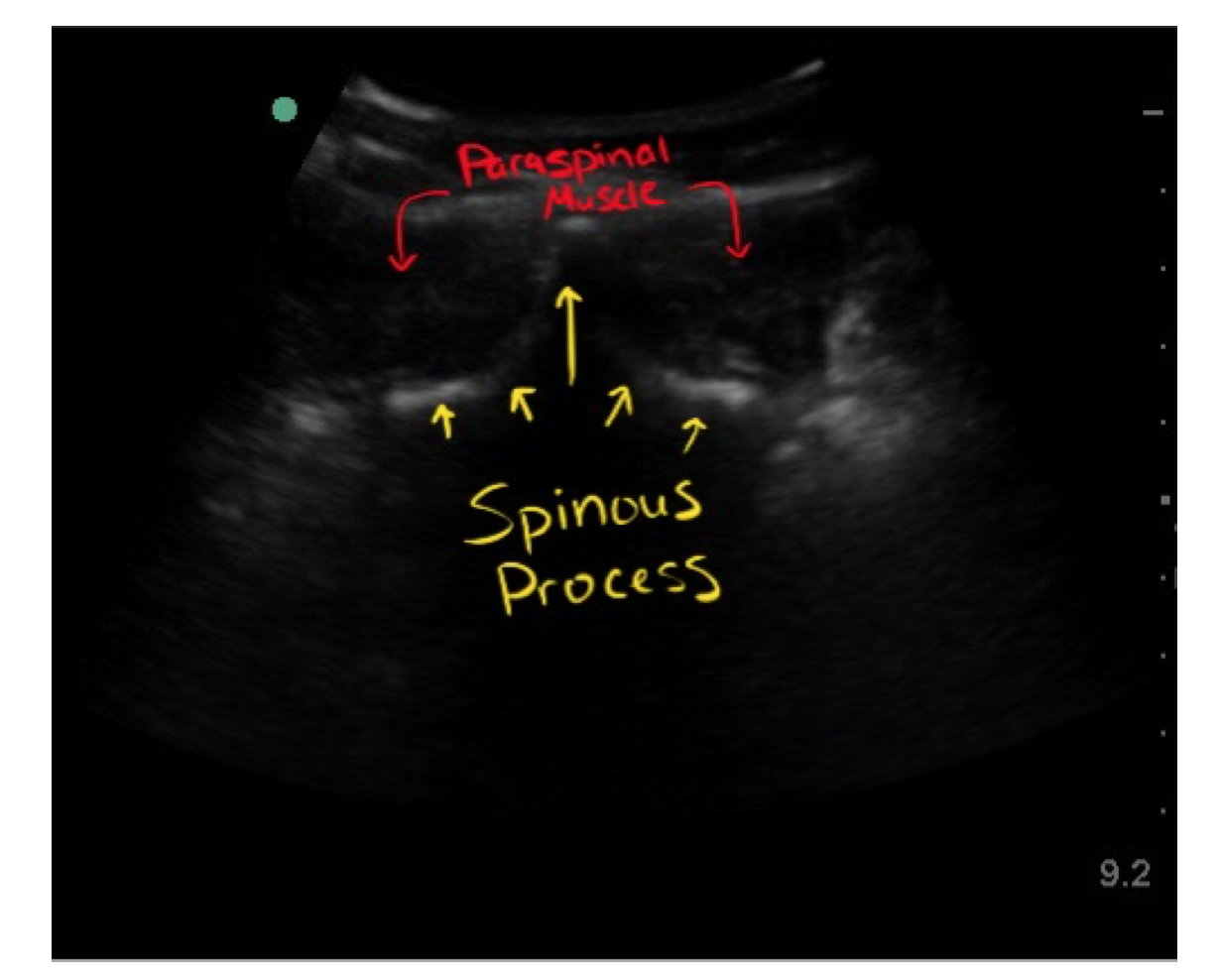
- Find the anatomic midline: with the probe in the transverse orientation (indicator to the patient’s right) and identify the spinous process. It will appear as a hyperechoic rim with posterior shadowing. Position the spinous process in the middle of your screen and use a marker on the skin to indicate the anatomic midline.
- Identify the intervertebral space: with the probe in the sagittal orientation (indicator cephalad) and maintaining midline using your previous mark (see above), identify the spinous processes and slide your probe slightly up or down until you are between two spinous processes. Position the intervertebral space in the middle of your screen and make a horizontal mark.
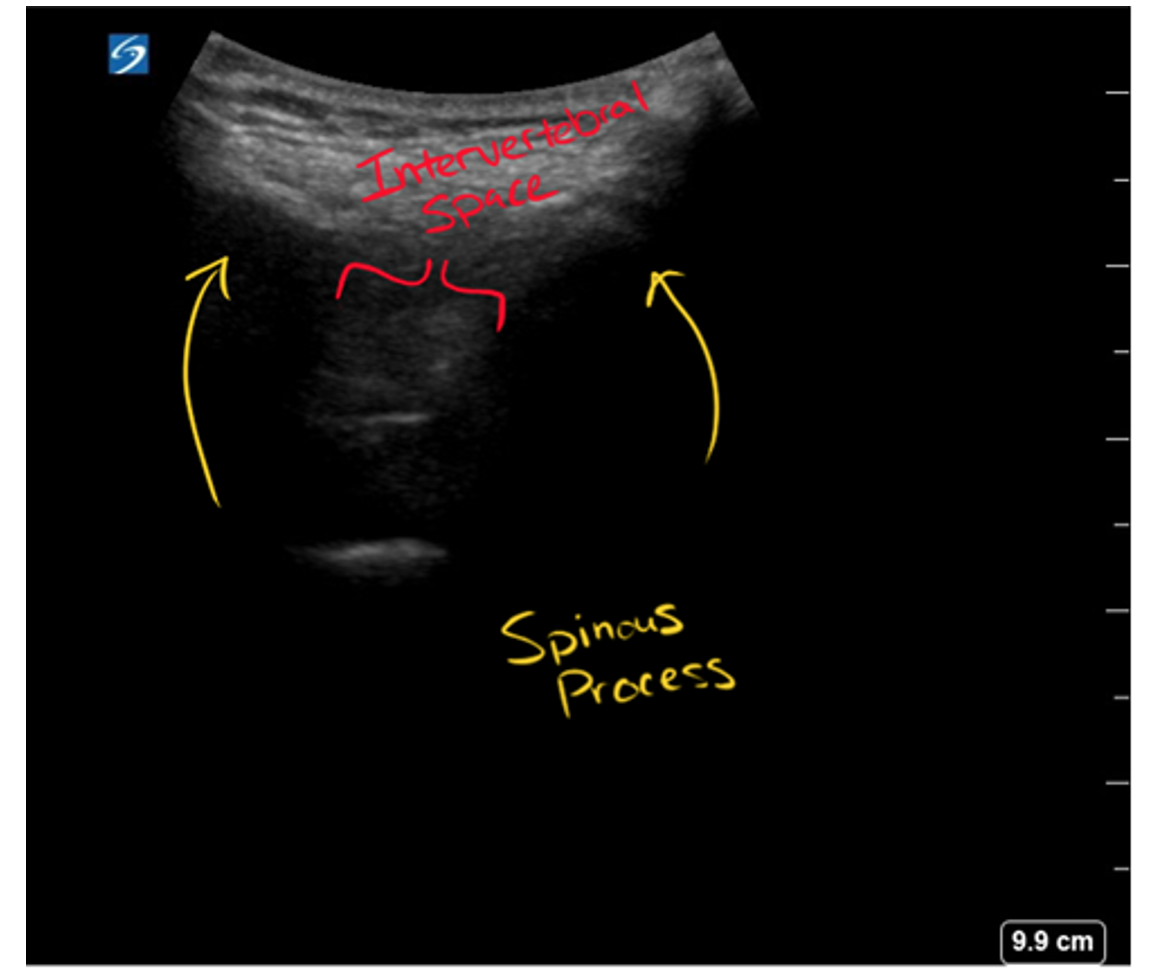
- Estimate depth of needle insertion: after identifying the spinous processes and intervertebral space in the sagittal view, you can increase the depth and the gain to view the mixed echogenicity soft tissue and ligaments, and then see the hypoechoic subarachnoid space underneath the dura mater.
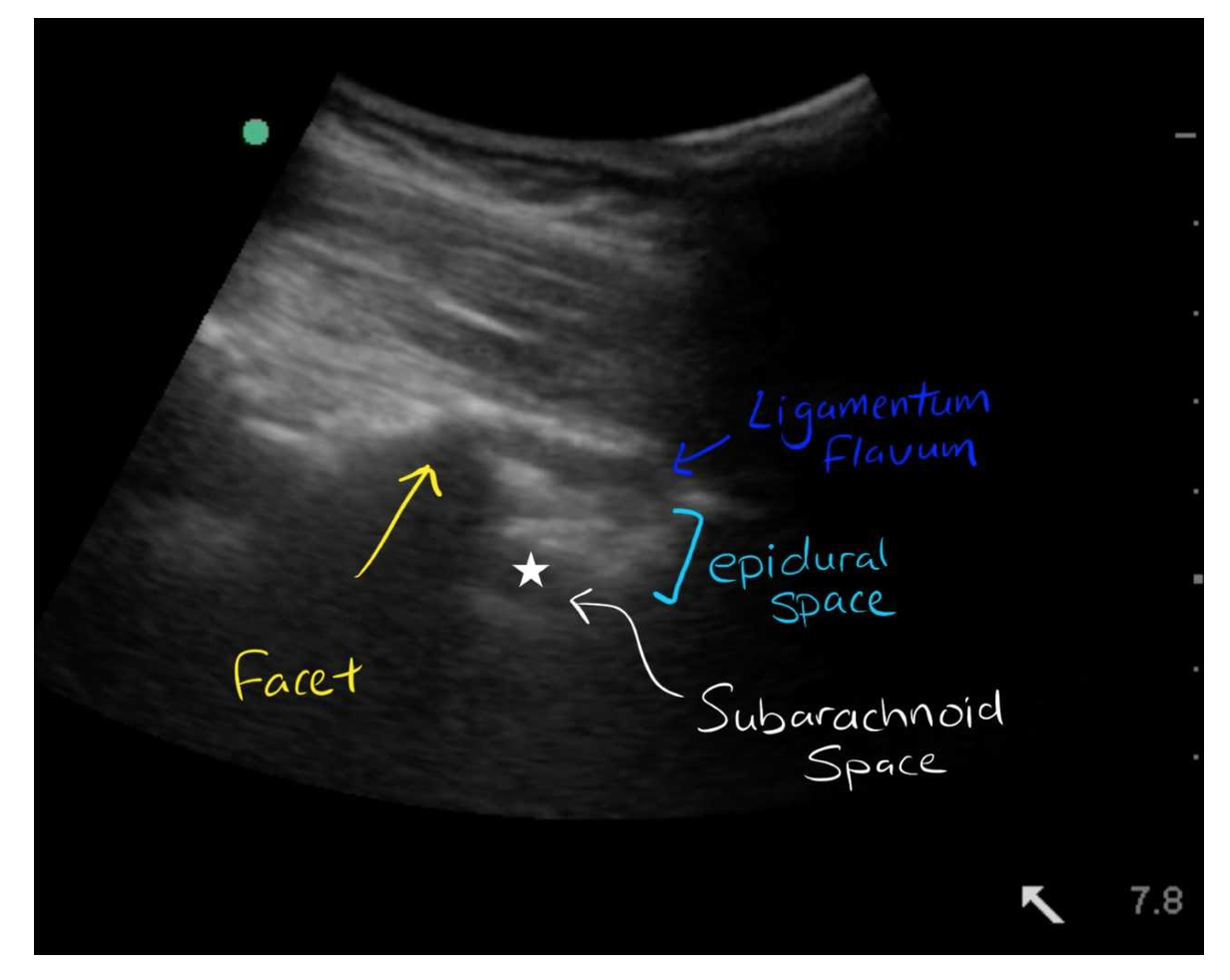
- Identify your space for needle insertion: connect the marks/lines you made for the anatomic midline and the intervertebral space. The intersection will be the optimal needle insertion site. Proceed with the LP.
Supporting Literature:
The use of ultrasound guidance for LPs has been studied extensively. Here are a handful of these studies and their conclusions:
Nomura et al., 2007 (randomized controlled trial)
- 46 patients – 22 in palpation landmark (PL) group and 24 in ultrasound landmark (UL) group
- Reported LP failure in 6/22 patients in PL group, 1/24 in UL group
- There were 12 obese patients in study: LP failure in 4/7 PL patients, 0/5 UL patients
- No statistical differences in the number of attempts, traumatic LPs, patient comfort, or procedure length
Ferre and Sweeney, 2007 (prospective cohort study)
- 2 operators, 76 patients – investigated visualization time for 5 anatomical structures (spinous processes or laminae, ligamentum flavum, dura mater, epidural space, subarachnoid space).
- High-quality images obtained in <1 minute in 87.9% of scans conducted and in <5 minutes in 100% of scans
Ferre et al., 2009 (pilot study)
- Used ultrasound as means of identifying site of skin puncture and needle depth needed to access the subarachnoid space
- CSF obtained in 36/39 patients in the first interspinous space attempted
Peterson et al., 2014 (randomized controlled trial)
- 50 patients in the landmark group and 50 patients in the ultrasound group
- Primary outcome: number of needle insertion attempts and LP success
- Secondary outcomes: pain associated with procedure, time to perform procedure, # traumatic taps, patient satisfaction
- Reported no significant differences in primary and secondary outcomes
Evans et al., 2018 (randomized controlled trial)
- Operators were residents in an emergency medicine residency program; underwent training on both landmark and ultrasound-guided techniques
- 35 patients total: 18 in landmark group and 17 in the ultrasound group
- Primary outcome: LP success in obtaining CSF
- No significant difference between groups
- Secondary outcomes: number of attempts, time to CSF flow, pain
- No significant differences in time to obtain CSF or mean pain scores
- Significant decrease in number of attempts—1.54 times more attempts in the landmark group and up to 1.7 times more attempts when adjusted for BMI
Gottlieb et al., 2019 (meta-analysis)
- Included 12 pediatric and adult studies (n=957 patients; 505 adults and 452 children)
- Primary outcome: LP success in obtaining CSF
- Ultrasound guided LP was successful in 90% of patients versus 81.4% for landmark LP
- Odds ratio 2.22 in favor of the ultrasound guided group
- Secondary outcomes: rate of traumatic LPs, time to procedural success, number of needle passes, patient pain score
- Fewer traumatic LPs in US group (10.7% vs. 26.5%; OR 0.28)
- Ultrasound guided LP associated with shorter time to successful LP by approximately one minute. However, this did not account for time taken to perform ultrasound, which may be substantial.
- Also reported fewer mean needle passes, lower patient pain scores for ultrasound guided LP
Take Home Points:
If you are anticipating a more challenging LP, due to a patient’s body habitus or positioning limitation, operator comfort or experience, or other factors, consider using the ultrasound. Take the time to pick up the probe and map out your procedure. This can make an otherwise challenging and uncomfortable procedure less painful and more successful on your first attempt.
References:
Evans, David P., et al. “Comparison of Ultrasound-Guided and Landmark-Based Lumbar Punctures in Inexperienced Resident Physicians.” Journal of Ultrasound in Medicine, vol. 38, no. 3, 2018, pp. 613–620., https://doi.org/10.1002/jum.14728.
Duniec, L, et al. Anatomical landmarks based assessment of intravertebral space level for lumbar puncture is misleading in more than 30%. Anaesthesiol Intensive Ther 2013; 45:1–6.
Ferre, R M, et al. “Ultrasound Identification of Landmarks Preceding Lumbar Puncture: A Pilot Study.” Emergency Medicine Journal, vol. 26, no. 4, 2009, pp. 276–277., https://doi.org/10.1136/emj.2007.057455.
Ferre, Robinson M., and Timothy W. Sweeney. “Emergency Physicians Can Easily Obtain Ultrasound Images of Anatomical Landmarks Relevant to Lumbar Puncture.” The American Journal of Emergency Medicine, vol. 25, no. 3, 2007, pp. 291–296., https://doi.org/10.1016/j.ajem.2006.08.013.
Gottlieb, Michael, et al. “Ultrasound-Assisted Lumbar Punctures: A Systematic Review and Meta-Analysis.” Academic Emergency Medicine, 2018, https://doi.org/10.1111/acem.13558.
Margarido, Clarita B., et al. “The Intercristal Line Determined by Palpation Is Not a Reliable Anatomical Landmark for Neuraxial Anesthesia.” Canadian Journal of Anesthesia/Journal Canadien D’anesthésie, vol. 58, no. 3, 2010, pp. 262–266., https://doi.org/10.1007/s12630-010-9432-z.
Millington, Scott J., et al. “Better with Ultrasound.” Chest, vol. 154, no. 5, 2018, pp. 1223–1229., https://doi.org/10.1016/j.chest.2018.07.010.
Nomura, Jason T., et al. “A Randomized Controlled Trial of Ultrasound-Assisted Lumbar Puncture.” Journal of Ultrasound in Medicine, vol. 26, no. 10, 2007, pp. 1341–1348., https://doi.org/10.7863/jum.2007.26.10.1341.
Peterson, Michael A., and Jennifer Abele. “Bedside Ultrasound for Difficult Lumbar Puncture.” The Journal of Emergency Medicine, vol. 28, no. 2, 2005, pp. 197–200., https://doi.org/10.1016/j.jemermed.2004.09.008.
Peterson, Michael A., et al. “Ultrasound for Routine Lumbar Puncture.” Academic Emergency Medicine, vol. 21, no. 2, 2014, pp. 130–136., https://doi.org/10.1111/acem.12305.
Strony, Robert. “Ultrasound-Assisted Lumbar Puncture in Obese Patients.” Critical Care Clinics, vol. 26, no. 4, 2010, pp. 661–664., https://doi.org/10.1016/j.ccc.2010.07.002.
“How To: Ultrasound Guided Lumbar Puncture Procedure 3D Video.” https://www.youtube.com/watch?v=ndnZxAcNjdg&ab_channel=Sonosite
“Lumbar Puncture.” https://oxfordmedicaleducation.com/clinical-skills/procedures/lumbar-puncture/
“Lumbar Puncture with Ultrasound.” https://www.youtube.com/watch?v=rbbpwE_ijm0&ab_channel=BCEmergencyMedicineNetwork
sinaiem.org/foam/try-ultrasound-for-your-next-lumbar-puncture
www.emdocs.net/ultrasound-lumbar-puncture-maximize-first-pass-success-patients-large-body-habitus