Overview:
- Toxic gas exposure is a rare but potentially lethal event with possibility of mass casualties
- Historically, toxic gas was used as a weapon of war, but in the modern era most exposures are from industrial accidents, house fires, and natural disasters
- Keep toxic gas exposure in the differential for any patient with AMS, respiratory symptoms, bradycardia, hypotension, or neurological symptoms, especially if fire or industrial related exposure
- Patients exposed to toxic gas require prompt recognition and decontamination to prevent further injury to them and everyone else in the ED
- Toxic gas physiology matches chemistry of the gas molecule
- Many drugs can be aerosolized and exert their expected physiologic effect after being absorbed through the lung
- e.g. Carfentanyl was used by the Russian government to knock out kidnappers in a hotel taken hostage; many died, including both terrorists and bystanders (2002)
- Categorize toxic gases by effect on respiratory system
- Simple asphyxiants
- Chemical asphyxiants
- Caustics
Decontamination:
- Decontamination is always the first step with any potential environmental contaminant
- Ensure all medical providers are wearing appropriate PPE
- Isolate the patient in a decontamination area
- CUT off clothes instead of pulling them over the head to avoid further facial exposure
- Seal affected clothing in plastic bags for future decontamination
- Gently irrigate any exposed skin with warm water and soap
- Irrigate eyes with Morgan lens or similar equipment until normalization of ocular pH (7.0)
Simple Asphyxiants:
Pathophysiology:
- Simple asphyxiants kill by reducing or displacing the normal oxygen in ambient air below FiO2 21% and impair oxygen exchange in the alveoli when present at high enough concentrations
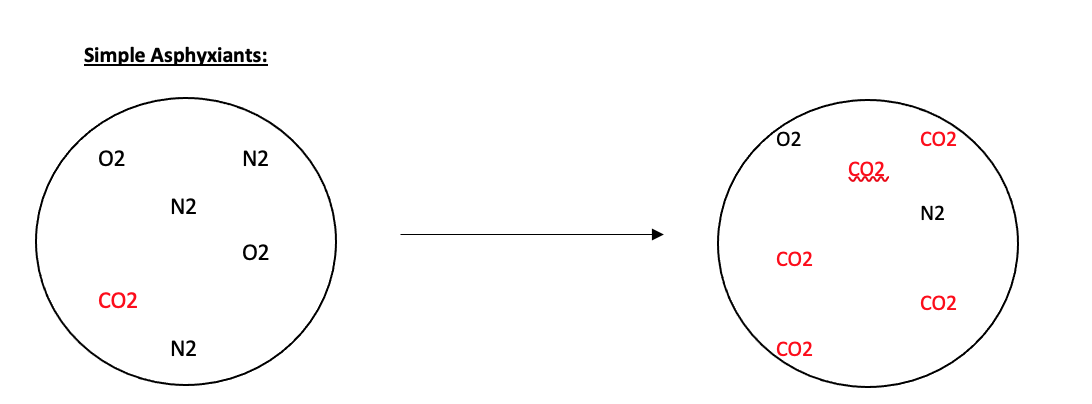
- There may be systemic effects from the particular gas, but primary cause of death is hypoxia
- Simple asphyxiants include methane, argon, nitrogen, helium, propane, and carbon dioxide
- Exposure typically occurs in an enclosed, poorly aerated space (e.g. garage or pool shed) where the gas can accumulate
- However, when volume is massive, outdoor exposure can be deadly
- In 1986, hundreds of thousands of tons of carbon dioxide gas were suddenly released from the bed of Lake Nyos in Cameroon, creating a cloud that rapidly descended down the valley onto nearby villages resulting in ~2000 deaths
Presentation:
- Simple asphyxiants are often odorless and inert, making exposure challenging to detect
- Case: a construction worker installing argon cooling lines in an enclosed space slowly tires then loses consciousness. A friend pulls him out; at first he’s blue and not moving, then rapidly regains consciousness when in clear air. He is awake and alert on arrival to the ED.
Management:
- Remove the exposure from the patient as quickly as possible
- Because simple asphyxiants are not systemic toxins, their effect is reversed by refilling the lungs with oxygenated air
- Death results from prolonged exposure without rescue leading to irreversible hypoxic tissue injury
- Supportive care in the ED (including ABCs and supplemental oxygen) and identification of the toxic gas to prevent repeated exposure
- Monitor for several hours
- Outpatient follow-up for potential delayed neurological sequelae
Chemical Asphyxiants:
Pathophysiology:
- Chemical asphyxiants prevent the body’s ability to properly utilize oxygen, either by interfering with hemoglobin oxygen transport or poisoning cellular respiration in the mitochondria (preventing the body from utilizing oxygen for ATP generation)
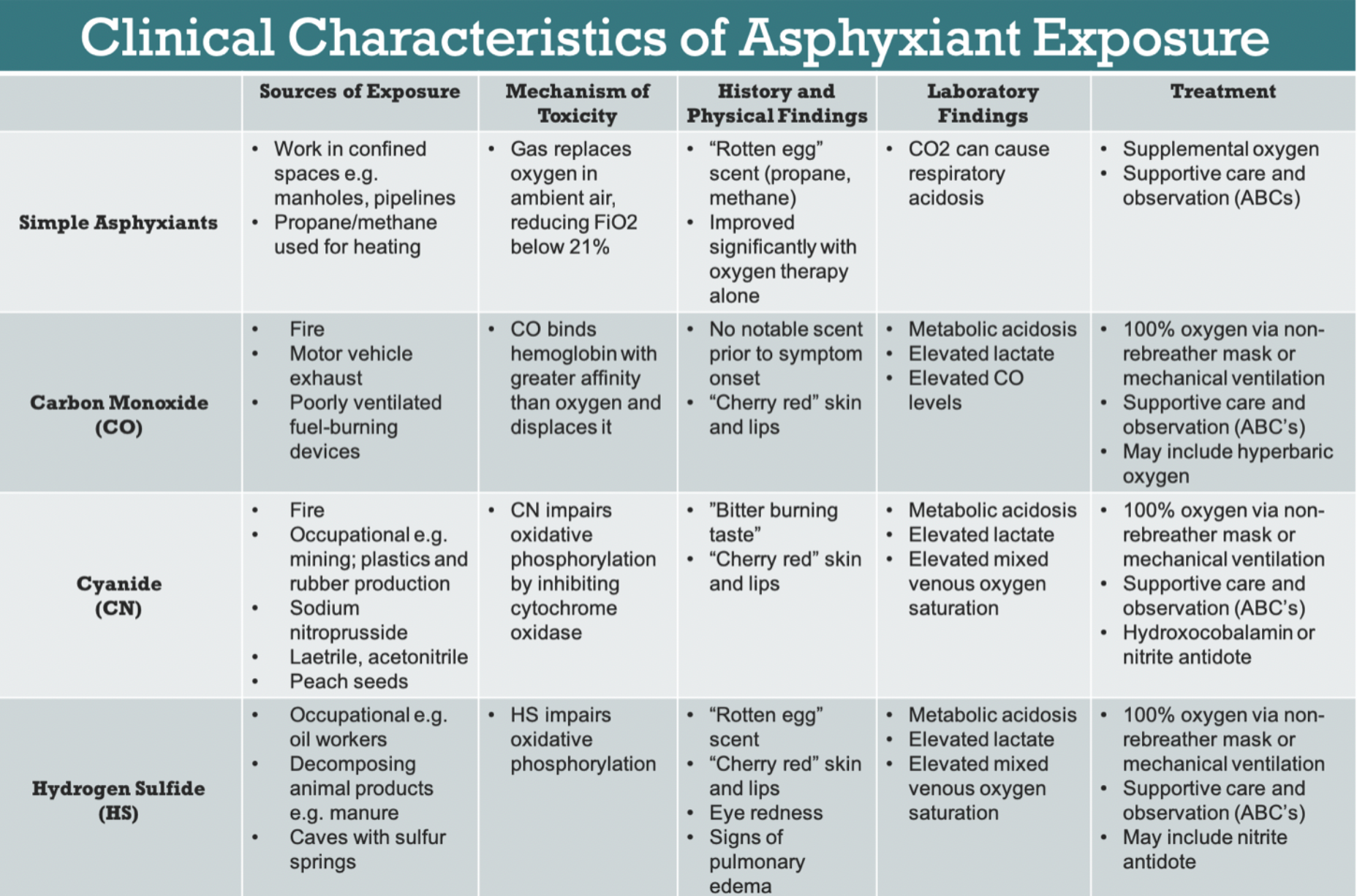
- Cyanide, carbon monoxide, and hydrogen sulfide are some of the most commonly encountered chemical asphyxiants
- Often called “knock down” gasses because they have a rapid, profound effect on consciousness and can “knock down” successive people on entering a room
Cyanide:
- Highly potent poisoner of cytochrome c oxidase, preventing oxidative phosphorylation and aerobic respiration
- Widespread tissue hypoxia ensues, rapidly progressing to multi-system organ failure and death
- Enhances NMDA receptor activity and induces oxidative stress in brain tissues, leading to neurotoxicity
- Outside of intentional poisoning, cyanide is most frequently encountered in fires where there is combustion of plastics
- Cyanide should be suspected in patients pulled from a fire who are critically ill or have a markedly elevated lactate, a sign of mitochondrial dysfunction
- Gold standard diagnosis does not exist but clues include AGMA, lactic acidosis >10, and normal pulse oximetry in combination with the history and exam
- Antidote of choice: hydroxycobalamin (dose: 5g reconstituted with 200mL 0.9% NS infused over 15 min, repeat if no improvement)
- Mechanism of action: hydroxycobalamin scavenges cyanide present in the blood and forms cyanocobalamin AKA vitamin B12
- Downside of hydroxycobalamin: interferes with all colorimetric blood analysis, as the dark red compound changes the light absorption spectrum used in these devices, so always draw blood for labs before administration of hydroxycobalamin
- In some jurisdictions, EMS protocols allow for administration of hydroxycobalamin in the field
- Cyanide Antidote Kit (CAK): amyl nitrite pearls (given as inhalation q30s of every minute until IV is established), followed by 300mg sodium nitrite IV over 5 min, and finally 12.5g sodium thiosulfate IV over 10-20 min
- CAK is an older antidote combination that is less commonly used as it induces methemoglobinemia, which can be lethal if administered to someone with concurrent carbon monoxide poisoning (a common scenario in house fires)
Hydrogen sulfide:
- Characterized by its “rotten egg” smell
- Mechanistically similar to cyanide
- Commonly encountered in industrial settings as well as natural ones, such as caves, volcanoes, and underground deposits of natural gas
- No proven antidote, but induction of methemoglobinemia through sodium nitrite and sodium thiosulfate has been used with good effect. In practice, exposure is often fatal on scene
- The methemoglobin scavenges H2S in the serum, creating sulfmethemoglobin and preventing further toxicity from the hydrogen sulfide
Carbon monoxide:
- Inhibits distal oxygen delivery due to higher binding affinity to hemoglobin than oxygen resulting in creation of carboxyhemoglobin
- Inhibits cytochrome oxidase and increases lipid peroxidation leading to oxidative stress
- Commonly found in fires as a product of carbon combustion
- Standard pulse oximetry cannot distinguish between carboxyhemoglobin and oxyhemoglobin so will be falsely reassuring
- Blood co-oximetry can measure carboxyhemoglobin levels
- Nonsmokers have carboxyhemoglobin levels ~3% or less
- Smokers can have carboxyhemoglobin levels 10-15%
- Initial management is application of 100% oxygen through nonrebreather or high flow
- Hyperbaric oxygen therapy (HBOT) can be used to decreased long-term neurological dysfunction
- Mechanism: increases the dissolved blood oxygen levels many times higher than possible with atmospheric pressure; the increase in pO2 enhances tissue oxygenation, hastens dissociation of CO from Hg, and improves restoration of mitochondrial function
- Consult toxicology or poison control center to discuss the need for hyperbaric oxygen therapy, but generally limited to presence of CO level >15% in pregnancy, CO level >25% in nonpregnancy, coma, neurological deficits, or myocardial ischemia
https://www.emra.org/emresident/article/asphyxiants/
Irritants:
- Cause mucosal and pulmonary irritation
- Timing and severity of effect depending on the chemical structure of the gas
Highly water-soluble gasses:
- Rapidly dissolve in the mucosal surfaces of the mouth, eyes, and oropharynx, causing immediate pain and coughing
- People exposed typically remove themselves as soon as possible, which often limits exposure
- Symptoms are proportional to extent of exposure, with severe cases leading to chemical burns in the oropharynx and airway
- Examples: Chloramine (bleach + ammonia, common household cleaners), capsaicin, sulfur dioxide
Poorly water-soluble gasses:
- Penetrate deep to the alveoli in gaseous form
- Gas molecule reacts with functional groups on alveoli leading to progressive inflammation and ARDS
- Do not cause immediate irritation, peak effect is typically 24 hours after exposure
- People often do not remove themselves from the source leading to prolonged exposure
- May even smell pleasant – Phosgene gas used in WWI smells like freshly mown hay
Management:
- Decontamination is first and foremost; irritants will cause continuous pain and inflammation until removed
- Supportive care with ABCs; severe airway or pulmonary inflammation may require intubation and mechanical ventilation
Summary:
Toxic gas exposures are rare but potentially lethal events. Prompt recognition and appropriate steps by out-of-hospital providers are mainstays of treatment. Emergency department management focuses on decontamination and supportive care, while antidotes are available for certain chemical asphyxiants. All management of toxic gas exposures should be performed in conjunction with your local poison control and department of health, as they pose unique risks to patients, providers, and the entire community.
References:
Nelson, L. (2019) Goldfrank’s Toxicologic Emergencies (11th ed). McGraw-Hill Medical.
- Charlton, N. and Kirk, M., Smoke Inhalation, pp 1640-1647
- Nelson, L. and Odujebe, O, Simple Asphyxiants and Pulmonary Irritants, pp 1651-1660.
- Tomaszewski, C., Carbon Monoxide, pp 1663-1672
- Holstege, C. and Kirk, M., Cyanide and Hydrogen Sulfide, pp 1684-1692
Toxicological Profiles | ATSDR. (n.d.). Agency for Toxic Substances and Disease Registry. Retrieved January 7, 2022, from https://www.atsdr.cdc.gov/toxprofiledocs/index.html?id=72&tid=19
Dries DJ, Endorf FW. Inhalation injury: epidemiology, pathology, treatment strategies. Scand J Trauma Resusc Emerg Med. 2013 Apr 19;21:31. doi: 10.1186/1757-7241-21-31. PMID: 23597126; PMCID: PMC3653783.
https://www.emra.org/emresident/article/asphyxiants/