Background
Definition: Infection endocarditis (IE) = Inflammation of the endothelium of the heart, heart valves (or both) (Osman 2013)
Epidemiology
- Annual incidence = 5-7 cases per 100,000 (Fraimow 2013)
- 40,000 to 50,000 new cases in the US per year. Average hospital charges in excess of $120,000 per patient (Bor 2013)
- Slightly higher male predominance (1.5:1 – 2:1) (Moreillon 2010)
- In-hospital mortality of 14–22% and 1-year mortality of 20-40% (Gomes 2017, (Habib 2006)
- Before antibiotics and surgery it was almost universally fatal (Aretz 2010, Osman 2013)
Pathophysiology (Moreillon 2010, Faza 2013, Tan 2014, Osman 2013, Kokowski 2018)
- The normal, undamaged valve endothelium is very resistant to colonization and infection by circulating bacteria
- Micro-trauma (caused by turbulent flow, intracardiac devices, etc) or chronic diseases (rheumatic heart disease, congenital heart disease, prosthetic valves, previous IE) can cause damage to the endothelium
- Damage to endothelium produces a fibin and platelet sterile thrombus. Microbes can seed that thrombus during transient episodes of bacteremia, fungemia and viremia
Risk factors (Faza 2013, Moreillon 2010).
- Diseased/damaged heart (highest risk)
- IV drug use (IVDU)
- Low immune function –
- Poor oral hygiene. (Faza 2013)
- Nosocomial
Diagnosis
- History
- Attention to any preexisting cardiac pathology or clues suggesting a recent source of bacteremia, such as IV drug use, indwelling intravascular catheters, or invasive procedures (Kokowsky 2018)
- Non-specific symptoms are common (Fraimow 2013, Kokowsky 2018)
- Fever is seen in 85%
- Malaise is seen in 80%
- Other constitutional symptoms: Weakness, arthralgias, weight loss (Faza 2013)
- Signs
- Pts more likely to present atypically (without fever and other systemic findings): Elderly, immunocompromised, and those with right heart endocarditis. (Tan 2014)
- Systemic Signs
- Can present with bacteremia/sepsis of unknown cause
- Can present in fulminant septic and cardiogenic shock. (Fraimow 2013)
- Cardiac manifestations
- Cardiac Murmur
- New cardiac murmur: 50% of presentations (Tan 2014)
- Regurgitant murmurs most common
- Early in the disease they may not have a murmur (even though eventually they almost invariably will have a murmur) (Kokowsky 2018)
- Atrioventricular (AV) nodal conduction abnormality (Faza 2013)
- Prolonged PR interval
- Heart block
- Heart failure from valvular insufficiency, valvular stenosis, rupture of an infected fistulous tract, and conduction system abnormalities. (Aretz 2010)
- Valvular insufficiency (Aretz 2010)
- Most common complication of IE
- Caused by either destruction of the valve by tears or penetrations, or loss of structural support by tethering of the chordae or the valve ring
- Cardiac Murmur
- Embolic manifestations
- Often is proximate cause of presentation to ED. (Moreillon 2010)
- Embolic complications may occur at any time during the course of IE (Fraimow 2013)
- Overall incidence of 20% to 50%
- Lower risk once antibiotics started, but can still occur
- Factors with Increased risk for thromboembolic events: (Baddour 2005)
- S. aureus, Candida, HACEK organisms, and Abiotrophia
- Vegetation size >10 mm
- Vegetation mobility
- Mitral valve involvement
- Renal emboli
- Emboli to kidneys can lead to abscess formation, ischemia, and infarction, presenting as flank pain, pyuria, or hematuria. May be confused with simple pyelonephritis, urinary tract infection or ureterolithiasis. (Osman 2013), Faza 2013)
- Can lead to “flea-bitten” appearance of cortex with focal segmental necrosis of the glomerular tuft (Aretz 2010)
- Pulmonary emboli
- More common in IVDU; tricuspid valve is almost always involved; pulmonic valve infection occurs in<5% of patients (Tan 2014)
- Pulmonary emboli happen exclusively with right sided IE unless there is concurrent left-sided endocarditis or paradoxical embolism (Tan 2014)
- Can be both septic emboli and “bland fibrin-platelet excrescences” that can lead to pulmonary infarction and septic pulmonary abscesses. (Aretz 2010)
- Can also present with pneumonia.
- Cerebral emboli
- Occurs in approximately 20% of pts with IE; mortality rate of 40% (Aretz 2010)
- CNS events (most commonly embolic events) are the second leading cause of death
- Neurologic manifestations occur in up to 40-50% of cases and are the second leading cause of death in IE. Complications include stroke, arteritis, abscesses, aneurysms, encephalomalacia, cerebritis and meningitis. (Moreillon 2010, Heiro 2000, Baddour 2005, Fraimow 2013)
- Eye emboli
- Conjunctiva
- While not sensitive or specific, painless conjunctival/subconjunctival hemorrhages can occur. (Osman 2013)
- Retina
- Microemboli to the eye vasculature can lead to visual field cuts (including monocular blindness). (Osman 2013)
- Ophthalmologic exam can reveal retinal hemorrhages and Roth spots (Aretz 2010)
- These spots are not pathognomonic for IE, but should raise suspicion for this disease (Osman 2013)
- Conjunctiva
- Splenic emboli
- Can cause flank pain or diaphragmatic irritation, or can be symptomatically silent (Faza 2013)
-
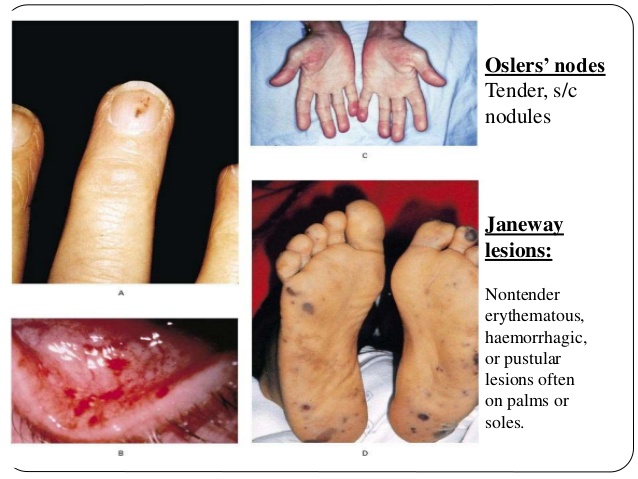
Cutaneous Findings in Endocarditis
Peripheral (skin) emboli
- Seen in 5-15% of IE patients. (Gomes 2016).
- Janeway lesions
- Painless hemorrhagic lesions on the feet and hands Marrie 2008.
- Caused by septic microemboli to skin with dermal microabscess formation Gomes 2016.
- Osler nodes
- Tender nodular erythema in the pads of the fingers and toes, thenar eminences, sides of the fingers, and the skin of the lower part of the arm Marrie 2008.
- Involvement of the glomus body and its specific nerve supply are thought to cause the pain seen in Osler’s lesions (Gunson 2007)
- Much of the literature reports that these lesions are due to immune complex deposition (Aretz 2010) or allergic vasculitis (Moreillon 2010), but more recent literature suggests etiology may also be microemboli and microabscess formation Gomes 2016.
- Local pain usually precedes the appearance of lesions by a few hours. The nodes may last from a few hours to several days, and leave no sequelae Gomes 2016.
- Janeway lesions and Osler’s nodes are not exclusive to endocarditis
- Seen in bacteremia without endocarditis, infected intravascular graft and in systemic lupus erythematosus Marrie 2008.
- Splinter hemorrhages
- Non-blanchable, reddish-brown to black linear lesions that are 1–3 mm in length that appear under the nail plate Haber 2016
- Lesions also described in scleroderma, trichinosis, SLE, rheumatoid arthritis, psoriasis, antiphospholipid syndrome, hematological malignancy, tyrosine kinase inhibitors, renal failure and trauma (Chong 2016, Haber 2016)
- Duke criteria
- Initially described 1994, then modified in 2000 (Li 2000)
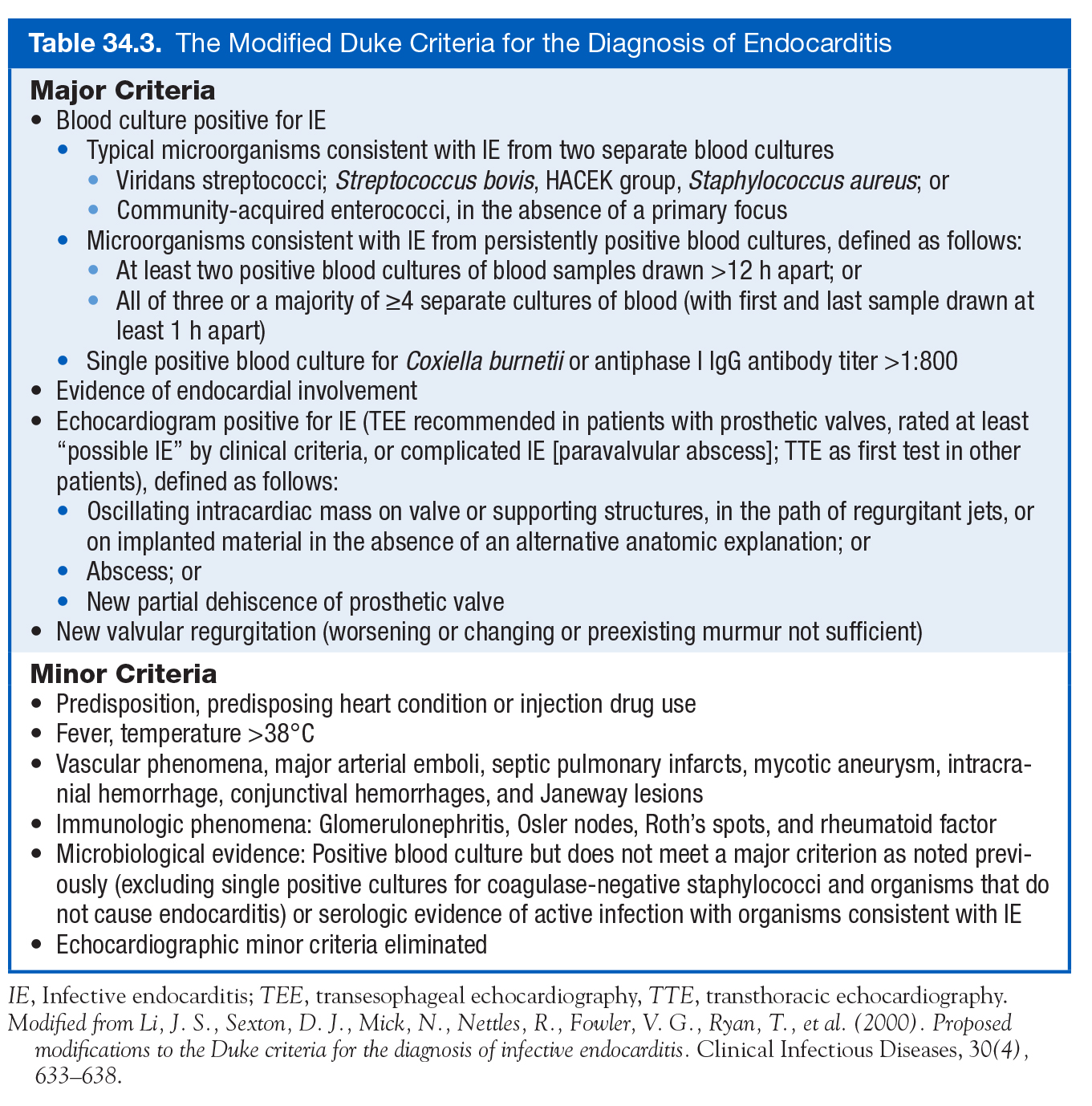
Modified Duke Criteria (Cardiology Secrets)
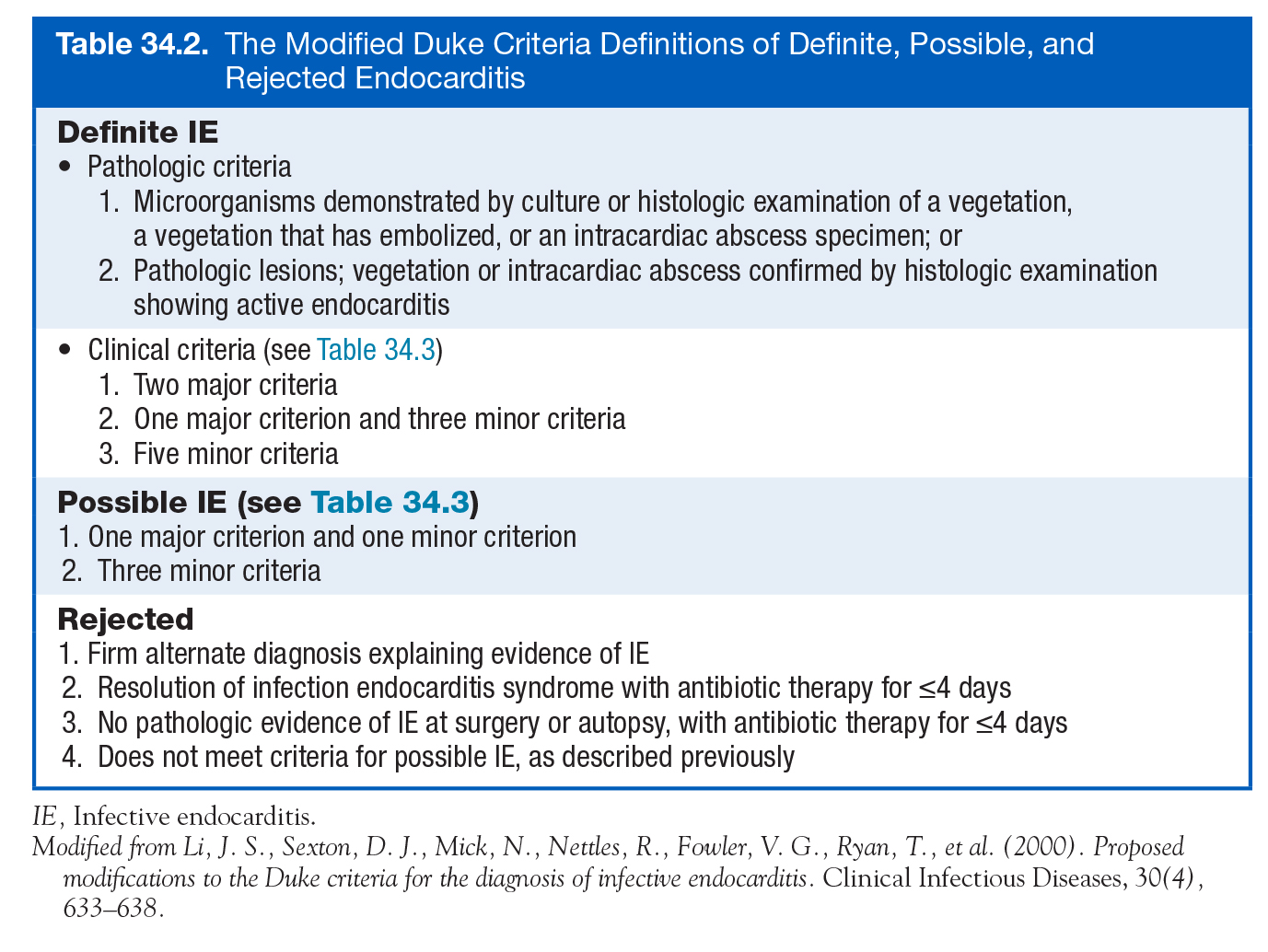
Modified Duke Criteria (Cardiology Secrets)
- Labs
- White Blood Cell (WBC) Count
- Neither sensitive nor specific for IE
- Can be normal, elevated or depressed in the setting of IE
- Elevated in around 50% of IE patients (Osman 2013), (Kokowsky 2018)
- Urinalysis (UA)
- Around 50% may have hematuria (due to embolism to kidneys) (Kokowsky 2018)
- CRP/ESR
- Sensitive, but not specific (Osman 2013)
- Elevated in 90–100% of cases (Moreillon 2010)
- Immune complexes (or rheumatoid factor) are present in up to 50% of patients after 6 weeks of infection (Moreillon 2010)
- Blood cultures
- 3 sets of cultures should be obtained.
- Many textbooks state that the 3 blood cultures should be obtained with at least 1 hour in between sets and before antibiotics. However, there is evidence that the volume of blood obtained is more important (Lamy 2016)
- If patient is unstable or acutely ill, blood culture sets can be obtained over a period of 5-20 minutes and antibiotics given as quickly as possible (Faza 2013, Osman 2013)
- Common pathogens (Moreillon 2010)
- Two sets will reveal the organism in 90%
- Three sets in up to 98% of cases
- Blood culture–negative IE occurs in 10-20% of cases (Aretz 2010, Tan 2014)
- Blood cultures should be held for 14 to 21 days before being labeled negative to identify fastidious organisms (Osman 2013)
- Previous abx therapy can lead to false negative results. (Osman 2013)
- Serology
- Common tests include the ELISA, complement fixation and indirect immunofluorescence for Chlamydia species, agglutination test for Brucella melitensis, indirect fluorescence for Legionella pneumoniae, ELISA for Mycoplasma pneumoniae. (Moreillon 2010)
- PCR of specific organisms (such as Coxiella, Brucella, bartonella, legionella, chlamydia. (Osman 2013)
- White Blood Cell (WBC) Count
- EKG
- Does not help in diagnosis but can detect complications
- Most common EKG findings: unremarkable or sinus tachycardia. (Osman 2013)
- Complications
- Acute myocardial infarction (secondary to coronary artery involvement)
- Conduction abnormalities: Complete heart block, atrioventricular block, and bundle branch blocks
- CXR
- No specific finding for IE
- Multiple bilateral pulmonary infiltrates might be a clue that septic emboli may be present (Osman 2013)
- CT scan
- Good for looking at complications in the chest, abdomen and pelvis (abscesses, pseudoanuerysms, distal infarctions/seeds, septic emboli) (Faza 2013)
- Abscesses can lead to fistulas between the left and right heart. (Aretz 2010)
- Transthoracic echo (Faza 2013, Fraimow 2013, Cahill 2017)
- Performance characteristics when TEE used as gold standard (Bai 2017)
- (+) LR: 9.5
- (-) LR: 0.42
- Useful to find cardiac complications (such as ventricular size/function, hemodynamic severity of valve lesions, etc)
- Detects 70% of vegetations larger than 6 mm; 25% of vegetations less than 5 mm
- TTE is not considered a sensitive test when a PVE or intracardiac device is present
- Up to 30% of patients with subsequently proven IE are labeled as “possible” due to equivocal or negative findings on echocardiography or blood cultures
- Performance characteristics when TEE used as gold standard (Bai 2017)
- Transesophageal echo (Habib 2015)
- Performance characteristics in NVE
- (+) LR: 9.6
- (-) LR: 0.04
- Superior to TTE for detection of complications, such as perforations, abscesses, and fistulae (Faza 2013, Cahill 2017)
- Performance characteristics in NVE
Microbiology of Infective Endocarditis
- Around 80% of IE cases are caused from gram positive organisms. (Moreillon 2010)
- S. aureus
- The most common organism implicated in IE (Vogkou 2016)
- Patients usually sicker and get sick faster
- Mortality rate has been reported to be as high at 47% (Moreillon 2010, Fraimow 2013)
- Can affect normal and abnormal valves (Osman 2013)
- S. viridans
- Effects only damaged valves (Osman 2013)
- Mitral and aortic valves are most commonly affected in cases of NVE, accounting for 90% to 95% of cases. (Tan 2014)
- S. epidermidis
- Associated with PVE, usually within 1 months (Osman 2013)
- S. bovis – Associated with GI course, more common in elderly
- S. pnuemoniae (Osman 2013)
- Acute fulminant illness
- Often effects the aortic valve
- High risk for perivaluvular abscess and pericarditis
- S. aureus
- P. aeruginosa – Sicker patients and IVDU (Osman 2013)
- Culture negative endocarditis:
- The most common cause of culture-negative endocarditis is prior antibiotics
- Other causes include fastidious organisms (HACEK group, Legionella, Chlamydia, Brucella, certain fungal infections, etc.) and noninfectious causes.
- HACEK = Hemophilus spp., Actino- bacillus spp., Cardiobacterium hominis, Eikenella corrodens, and Kingella kingii.
- Definition: Slow- growing gram-negative bacilli can require several weeks before they are detected in culture.
- Comprised 1-3% of all IE cases, with a mean delay in diagnosis from 1-3 months Revest 2016.
- Part of normal oral microbiota (some are also urogenital) Revest 2016.
- The HACEK group of organisms may cause large vegetations and large-vessel embolism. Diagnose those by serologic testing and polymerase chain reaction (PCR)-based testing
- HACEK = Hemophilus spp., Actino- bacillus spp., Cardiobacterium hominis, Eikenella corrodens, and Kingella kingii.
Management
- ED management:
- Address issues with airway, breathing and circulation if present
- Antibiotics
- Standard Empiric Regimen: Vancomycin 15 mg/kg and Ceftriaxone 2 grams
- Addition of amino glycoside
- Some sources recommend adding aminoglycoside (such as gentamycin), especially for enterococcal infections
- Increasing evidence that aminoglycosides may cause harm without clear benefit
- Multiple RCT’s and observational studies have found increased renal dysfunction with gentamycin without increased efficiency of clearing IE (Fowler 2006, Fernandez 2013, Galvalda 2007)
- Duration of antibiotics:
- Traditionally, prolonged (4–6 weeks) treatment is mandatory to kill dormant bacteria clustered in the infected foci (Moreillon 2010)
- Some sources suggest that in uncomplicated NVE with normal renal function may need antibiotic for as little as 2 weeks. Cahill 2017.
- Surgery
- There appears to be a trend towards better outcomes when surgery is done instead of medical management
- Particularly true in left sided endocarditis and s. aureus endocarditis (Fraimow 2013)
- No randomized controlled trials on medical vs. surgical management (Fraimow 2013)
- In IVDU associated IE, medical management usually is effective (Tan 2014)
- A recent meta-analysis of 32 studies that included 2,636 pts found that valve reoperation for PVE had a lower mortality and similar rate of PVE endocarditis compared to medical management Mihos 2017
- Broad indications for surgery
- Refractory CHF
- Cardiogenic shock due to valvular insufficiency
- Persistent infection despite optimal antimicrobial therapy
- Fungal or other difficult-to-treat organisms
- One or more emboli during the first weeks of antimicrobial therapy
- Balvular complications of dehiscence, perforation, fistula, and large perivalvular abscesses (Baddour 2005, Osman 2013)
- Other potential indications for surgery in patients with IE are failure of antibiotic therapy, vegetations larger than 10 mm on echocardiography, fungal endocarditis, early prosthetic valve endocarditis (within the first 2 months after surgery), and recurrent embolization despite medical therapy. (Osman 2013)
- R sided endocarditis generally doesn’t get surgery. In part because there is a high recurrence rate of IE in IVDU
- ESC 2015 guidelines when to do surgery on RH endocarditis: Right HF due to severe tricuspid regurgitation (TR) not responding to diuretics, Tricuspid valve vegetations greater than 20 mm that persist after recurrent pulmonary emboli with or without concomitant right HF, IE caused by organisms that are difficult to eradicate or bacteremia for at least 7 days despite adequate antimicrobial therapy
- There appears to be a trend towards better outcomes when surgery is done instead of medical management
- Anticoagulation
- Anticoagulation is not indicated for patients with endocarditis. It prevents neither the formation nor the embolization of vegetations (Osman 2013)
- Patients already on anticoagulation when endocarditis occurs:
- Anticoagulation has been considered to be relatively contraindicated in active endocarditis because of the risk of hemorrhagic CNS events (Baddour 2005, Snygg-Martin 2011)
- However, data from a recent case cohort study suggest that risk of warfarin therapy in acute left-sided native valve endocarditis has been overestimated. (Snygg-Martin 2011)
- If patients are already on anticoagulation before the endocarditis (such as a patient with prosthetic valve), they should probably be switched to something like heparin. If CNS events occur, should discontinue (Osman 2013)
- Disposition
- Admit for IV antibiotics. Early surgical consultation.
- Traditionally, patients needed to stay in the hospital for their entire course (>6 weeks), but now there’s options to be discharged with abx after a few days/weeks in the hospital (Kokowsky 2018, Cahill 2017.)
Take Home Points
- Endocarditis is a disease with high morbidity and mortality
- Signs and symptoms can be very non-specific. Suspect the diagnosis in anyone with a new murmur, bacteremia without a clear focus, peripheral embolic phenomena or IVDU
- Diagnosis is usually based off of the Modified Duke criteria
- ED management includes empiric antibiotics covering the most common pathogens and consultation with surgery particularly if the patient has valvular insufficiency
Read More
- Hippo EM Podcast: Episode 05 – Endocarditis
- CrackCast: Episode 83 – Infective Endocarditis and Valvular Disease
- EmCrit: Podcast 166 – Endocarditis with David Carr
- EmDocs: Endocarditis – A Complicated Case
- Emergency Medicine Cases – Best Case Ever 32, Carr’s Cases – Endocarditis and Blood Culture Interpretation
- LITFL: Critical Care Compendium – Infective Endocarditis
References
- Osman S., Carter W: Endocarditis in Adams J.G. et al, Emergency Medicine Clinical Essentials ed 2. Philadelphia: Elsevier, 2013 (Ch) 62: p. 530-546
- Fraimow, H, Reboli A: Specific Infections with Critical Care Implications, in Parillo J et al (eds): Critical Care Medicine: Principles of Diagnosis and Management in the Adult, ed 4. Missouri: Mosby, 2013 (Ch) 54: p. 936-961
- Bor DH, Woolhandler S, Nardin R, Brusch J, Himmelstein DU. Infective endocarditis in the U.S., 1998-2009: a nationwide study. PloS one. 2013; 8(3):e60033. PMID: 23527296
- Moreillon, P. Endocarditis and Enteritis, in Cohen J (ed): Infectious diseases, ed 3. Missouri, 2010. (Ch) 47: p. 514-528
- Aretz, H., & Krandin, R: Cardiac Infections, in Krandin, R (eds): Diagnostic Pathology of Infectious Disease, ed 1. Philadelphia: Saunders, 2010, (Ch) 8. P. 189-213
- Faza, N., & Shah T: Endocarditis and Endocarditis Prophylaxis, in Levine, G (ed): Cardiology secrets, ed 4. Philadelphia: Saunders, 2013, (Ch) 34. p. 309-320
- Kosowsky, J., & Takhar S: Infective Endocarditis, Rheumatic Fever, and Valvular Heart Disease in Marx J et al (eds): Rosens Emergency Medicine: Concepts and Practice, ed 9. Philadelphia: Elsevier, 2018 (Ch) 73: p. 1000-1006
- Baddour LM, Wilson WR, Bayer AS. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005; 111(23):e394-434. PMID: 15956145
- Heiro M, Nikoskelainen J, Engblom E, Kotilainen E, Marttila R, Kotilainen P. Neurologic manifestations of infective endocarditis: a 17-year experience in a teaching hospital in Finland. Archives of internal medicine. 2000; 160(18):2781-7. PMID: 11025788
- Snygg-Martin U, Rasmussen RV, Hassager C, Bruun NE, Andersson R, Olaison L. Warfarin therapy and incidence of cerebrovascular complications in left-sided native valve endocarditis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2011; 30(2):151-7. PMID: 20857163
- Devlin RK, Andrews MM, von Reyn CF. Recent trends in infective endocarditis: influence of case definitions. Current opinion in cardiology. 2004; 19(2):134-9. PMID: 15075740
- Tan, C., & Rodriguez E: Endocarditis and Other Intravascular Infections, in Procop, G et al (eds): Pathology of Infectious Diseases, ed 1. Philadelphia: Saunders, 2014, (Ch) 11. P. 229-246
- Freedman LR, Valone J. Experimental infective endocarditis. Progress in cardiovascular diseases. 1980; 22(3):169-80. PMID: 388518
- Habib G. Management of infective endocarditis. Heart (British Cardiac Society). 2006; 92(1):124-30. PMID: 16365367
- Eiken PW, Edwards WD, Tazelaar HD, McBane RD, Zehr KJ. Surgical pathology of nonbacterial thrombotic endocarditis in 30 patients, 1985-2000. Mayo Clinic proceedings. 2001; 76(12):1204-12. PMID: 11761501
- Wilson W, Taubert KA, Gewitz M. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007; 116(15):1736-54. PMID: 17446442
- Cahill TJ, Baddour LM, Habib G. Challenges in Infective Endocarditis. Journal of the American College of Cardiology. 2017; 69(3):325-344. PMID: 28104075
- Selton-Suty C, Célard M, Le Moing V. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012; 54(9):1230-9. PMID: 22492317
- Fowler VG, Boucher HW, Corey GR. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. The New England journal of medicine. 2006; 355(7):653-65. PMID: 16914701
- Fernández-Hidalgo N, Almirante B, Gavaldà J. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating enterococcus faecalis infective endocarditis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013; 56(9):1261-8. PMID: 23392394
- Gomes RT, Tiberto LR, Bello VN, Lima MA, Nai GA, Abreu MA. Dermatologic manifestations of infective endocarditis. Anais brasileiros de dermatologia. 2016; 91(5 suppl 1):92-94. PMID: 28300907
- Gomes A, Glaudemans AWJM, Touw DJ. Diagnostic value of imaging in infective endocarditis: a systematic review. The Lancet. Infectious diseases. 2017; 17(1):e1-e14. PMID: 27746163
- Lamy B, Dargère S, Arendrup MC, Parienti JJ, Tattevin P. How to Optimize the Use of Blood Cultures for the Diagnosis of Bloodstream Infections? A State-of-the Art. Frontiers in microbiology. 2016; 7:697. PMID: 27242721
- Pasha AK, Lee JZ, Low SW, Desai H, Lee KS, Al Mohajer M. Fungal Endocarditis: Update on Diagnosis and Management. The American journal of medicine. 2016; 129(10):1037-43. PMID: 27267285
- Giamarellou H. Nosocomial cardiac infections. The Journal of hospital infection. 2002; 50(2):91-105. PMID: 11846535
- Pappas PG, Kauffman CA, Andes D. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009; 48(5):503-35. PMID: 19191635
- Revest M, Egmann G, Cattoir V, Tattevin P. HACEK endocarditis: state-of-the-art. Expert review of anti-infective therapy. 2016; 14(5):523-30. PMID: 26953488
- Gunson TH, Oliver GF. Osler’s nodes and Janeway lesions. The Australasian journal of dermatology. 2007; 48(4):251-5. PMID: 17956487
- Marrie TJ. Osler’s nodes and Janeway lesions. The American journal of medicine. 2008; 121(2):105-6. PMID: 18261495
- Mihos CG, Capoulade R, Yucel E, Picard MH, Santana O. Surgical Versus Medical Therapy for Prosthetic Valve Endocarditis: A Meta-Analysis of 32 Studies. The Annals of thoracic surgery. 2017; 103(3):991-1004. PMID: 28168964
- Vogkou CT, Vlachogiannis NI, Palaiodimos L, Kousoulis AA. The causative agents in infective endocarditis: a systematic review comprising 33,214 cases. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2016; 35(8):1227-45. pubmed
- Chong Y, Han SJ, Rhee YJ, Kang SK, Yu JH, Na MH. Classic Peripheral Signs of Subacute Bacterial Endocarditis. The Korean journal of thoracic and cardiovascular surgery. 2016; 49(5):408-412. PMID: 27734006
- Haber R, Khoury R, Kechichian E, Tomb R. Splinter hemorrhages of the nails: a systematic review of clinical features and associated conditions. International journal of dermatology. 2016; 55(12):1304-1310. PMID: 27420914
- Li JS, Sexton DJ, Mick N. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000; 30(4):633-8. PMID: 10770721
- Bai AD, Steinberg M, Showler A. Diagnostic Accuracy of Transthoracic Echocardiography for Infective Endocarditis Findings Using Transesophageal Echocardiography as the Reference Standard: A Meta-Analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2017; 30(7):639-646.e8. PMID: 28483353
- Habib G, Lancellotti P, Antunes MJ. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). European heart journal. 2015; 36(44):3075-128. PMID: 26320109
