Background
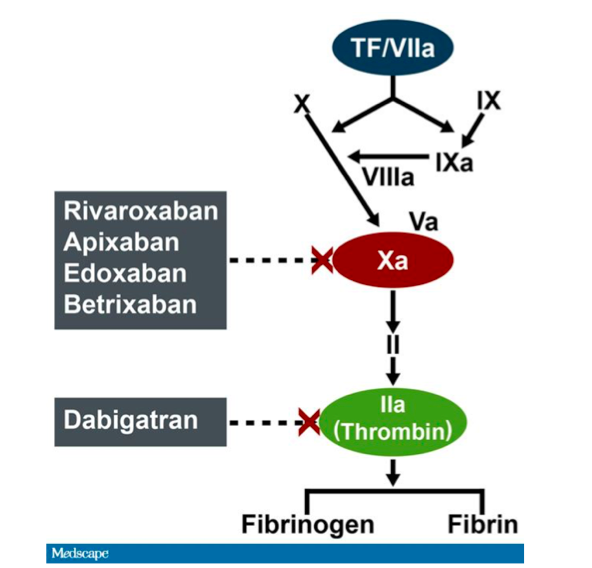
Dabigatran Site of Action (www.medscape.com)
The burdens associated with the use of traditional anticoagulants such as subcutaneous heparins and oral vitamin K antagonists (VKAs) have spurred the popularity of non-vitamin K oral anticoagulants (NOACs) for stroke prevention in patients with nonvalvular atrial fibrillation and for the prevention and treatment of venous thromboembolism. In spite of the conveniences associated with the use of these new anticoagulants, life-threatening bleeding can occur and there are no proven options for anticoagulation reversal for patients on these drugs.
Dabigatran, an oral thrombin inhibitor, is one of the NOACs currently in vogue. Boehringer Ingelheim, the pharmaceutical company that developed dabigatran, has now developed a monoclonal antibody fragment, idarucizumab, with the hope that it can serve as an antidote in patients with life-threatening bleeding on dabigitran. Idarucizumab has a binding affinity to dabigitran that is 350 times as high this NOACs affinity for thrombin. Phase I trails, sponsored by the same company, have been completed and have demonstrated that this antidote is potentially efficacious.
Clinical Question
In patients taking dabigatran, who present with serious bleeding or require urgent surgery/intervention, is idarucizumab safe and efficacious for the reversal of anticoagulation?
Population
All adults (≥ 18 years old) reported to have been taking dabigatran presenting with overt, uncontrollable, or life-threatening bleeding (Group A) OR who required surgery or other invasive procedure that could not be delayed for at least 8 hours (Group B)
Intervention
All patients were given 5 g of IV idarucizumab in two 2.5 g boluses 15 minutes apart.
Outcomes
Primary:Maximum percentage reversal of anticoagulant effect within 4 hours assessed by measurement of dilute thrombin time and ecarin clotting time.
Secondary:Reduction in the concentration of unbound dabigatran; restoration of hemostasis; suspected thrombotic events or deaths by 90 days.
Design
Multicenter, international, prospective observational cohort study
Excluded
< 18 years old, persons with contraindications to study medications including known hypersensitivity to the drug and its excipients; Group A – minor bleeding (e.g. epistaxis, hematuria) who can be managed with standard supportive care or no bleeding; Group B – Patients requiring a surgery or procedure which is elective or where the risk of uncontrolled or unmanageable bleeding is low.
Primary Results
Primary Results
- 90 patients enrolled at 184 sites in 35 countries between June 2014 and February 2015 with average age of 76.5 years
- 51 patients in group A (18 intracranial bleeds, 20 GI bleeds, 9 traumas, 11 “other”)
- 39 patients in group B (indications for surgery/invasive procedure varied but most common was bone fractures)
- 22 patients (11 from either group) were found to have dilute thrombin times deemed to be within normal limits (9 of these 22 also had normal ecarin clotting times) so they were excluded from efficacy analysis but were still given idaricuzumab and included in the study and safety analysis
Critical Findings
- Median maximum percentage reversal of the anticoagulant effect of dabigatran, assessed by both the dilute thrombin and ecarin clotting time, within four hours was 100% (95% CI, 100% to 100%)
- After first vial of idaricuzumab, concentration of unbound dabigatran was less than 20 ng/ml – a level that produces little or no anticoagulant effect – in all but one patient
- Of the 38 of 51 patients that could be assessed for hemostasis in Group A (difficult to assess hemostasis in ICH – 5 pts, GIB – 4 pts, intramuscular bleeding – 2 pts, pericardial bleeding – 1 pt, RP bleeding – 1 pt), median investigator-reported time to the cessation of bleeding was 11.4 hours
- In Group B, of the 36 of 38 patients who actually underwent invasive procedure, normal operative hemostasis reported in 92% (33/36)
- 5 patients experienced thrombotic events at 90 days (1 patient within 72 hours of drug administration)
Strengths
- Prospective, multicenter, international trial
- Minimal exclusions increasing applicability
- Follow-up was almost complete and appropriately long
Limitations
- Study focused on assessing pharmacokinetic data (improvement of dilute thrombin times and ecarin clotting times) as opposed to focusing on patient-centered outcomes (hemostasis, survival, etc.)
- Small number of patients limiting power
- No control group
- Non-blinded
- Possible selection bias – no data given regarding number of patients screened for enrollment
- Potential confounders (e.g. use of antiplatelet therapy) not addressed
Other Issues
- Potential for significant bias: The trial was funded by the manufacturer -Boehringer Ingelheim. Additionally, the drug company marketing the drug had a role in the study design. Many authors report a number of relevant financial conflicts of interest.
Author's Conclusions
“Idaricuzumab rapidly and completely reversed the anticoagulant activity of dabigatran in 88 to 98% of patients. There were no safety concerns among the 90 patients involved in this study.”
Our Conclusions
Idaricuzumab normalizes coagulation parameters in patients with life-threatening bleeds within minutes. Idarucizumab’s clinical effectiveness in achieving meaningful hemostasis and its safety cannot be confirmed based on this data.
Potential Impact To Current Practice
Currently, our only options for patients on dabigatran who present with life-threatening bleeding are blood products (PCC, FFP, Factor VIIa) to try to initiate reversal of the anticoagulation effect of this direct thrombin inhibitor and hemodialysis to facilitate its removal. In these desperate situations, having another agent, like idaricuzumab, that has been proven to improve coagulation parameters in patients taking dabigatran, is a welcome alternative. Although its true clinical efficacy and safety needs to be further elucidated, idaricuzumab can and will likely be used in hemodynamically unstable patients with life-threatening bleeding who are on dabigatran.
Bottom Line
Idaricuzumab improves coagulation parameters in patients on dabigitran who present with life-threatening bleeding or who require anticoagulation reversal for emergent invasive procedures. Further larger studies need to be pursued to investigate idaricuzumab’s impact on patient-centered outcomes, like hemostasis, survival, and functional outcomes, and to further assess its safety profile.
Read More
REBEL EM: Noreversaban?
The SGEM: One Thing Leads to Another – Idarucizumab for Dabigatran Reversal
EM Lit of Note: Let’s Reverse: Dabigatran