Background
Carbon monoxide is one of the most common causes of fatal poisoning through either intentional or unintentional exposure and accounts for almost 50,000 emergency departments per year. (Sircar 2015, PMID: 26032660) It is an odorless, colorless gas that is produced from relatively common sources such as vehicle exhaust, propane fueled heaters, wood/coal burning stoves and gasoline powered generators. Carbon monoxide has an affinity for hemoglobin ~200x that of oxygen and results in the formation of carboxyhemoglobin, a form of hemoglobin that does not participate in oxygen delivery, which has been thought to cause a relative anemia and tissue hypoxia. However, more recent literature suggests that circulating CO may have a direct role in causing tissue damage. (Gozubuyuk 2017, PMID: 28752154) Carbon monoxide poisoning manifests as a flu like illness in mild exposures (headache, dizziness, weakness, vomiting, chest pain) and more significant illness with larger exposures (loss of consciousness, arrhythmia, seizure, memory issues, movement/cerebellar issues and death).
At the time this landmark study was published in 2002, the standard of care for carbon monoxide poisoning was 100 percent normobaric oxygen through a facemask with experts often recommending hyperbaric oxygen therapy if patients had lost consciousness or had severe poisoning. Previous studies had suggested that 100% oxygen at normobaric pressure decreased the half-life of carboxyhemoglobin by 5 fold (5 hrs → 1 hr) and suggested that this was even more significant with at hyperbaric pressures (5 hrs → 24 minutes). (Buckley 2011, PMID: 21491385) The authors of this study noted that many patients with severe carbon monoxide poisoning tend to have cognitive sequelae and sought to evaluate what, if any effect, hyperbaric oxygen therapy had on these cognitive sequelae.
Clinical Question
What effect does hyperbaric oxygen have on the cognitive sequelae of carbon monoxide poisoning?
Population
Patients were enrolled from emergency departments in Utah, Idaho, and Wyoming who referred 98 percent of all patients with known carbon monoxide poisoning to LDS Hospital in Salt Lake City from November 1992 through February 1999. A total of 460 patients were assessed, 332 deemed eligible and 152 enrolled in the trial.
Intervention
Three hyperbaric chamber sessions occurring within a 24 hr period (see figure in “Read More” section.
Control
Three normobaric oxygen sham chamber sessions (one w/oxygen and 2 w/air) occurring within a 24 hr period
Outcomes
Primary outcomes: The incidence of cognitive sequelae six weeks after randomization.
Secondary outcomes: Neuropsychological test scores obtained after the third chamber session, including the testing performed six weeks after the carbon monoxide poisoning; self-reports of symptoms of carbon monoxide poisoning at six weeks; scores on the Geriatric Depression Scale, the Katz index of activities of daily living, and the SF-36 at two and six weeks; and the results on the neurologic examination after the third chamber session.
Design
Quadruple-blinded RCT with blocked, stratified randomization
Excluded
Inclusion: Patients were eligible for enrollment if they had a documented exposure to carbon monoxide (elevation of the carboxyhemoglobin level or the ambient carbon monoxide concentration) or an obvious exposure to carbon monoxide and if they had any of the following symptoms: loss of consciousness, confusion, headache, malaise, fatigue, forgetfulness, dizziness, visual disturbances, nausea, vomiting, cardiac ischemia, or metabolic acidosis (a calculated base excess lower than –2.0 mmol per liter or a lactate concentration higher than 2.5 mmol per liter). If the carboxyhemoglobin level was below 10 percent, the patient was eligible only if carbon monoxide poisoning was the only plausible diagnosis.
Exclusion: Patients were excluded if more than 24 hours had elapsed since the exposure to carbon monoxide had ended; if they were younger than 16 years of age; if they were moribund; if informed consent could not be obtained; or if they were pregnant. Additional patients chose not to enroll due to the cost, inconvenience, risk of complication or anxiety surrounding the treatment.
Primary Results
- For all 152 patients in the intention to treat population, cognitive sequelae were less frequent at six weeks in the hyperbaric-oxygen group (25 percent) than in the normobaric-oxygen group (46.1 percent).
- For the 147 patients with complete data on neuropsychological testing at 6 weeks out, there was fewer incidence of cognitive sequelae in the hyperbaric oxygen treatment group (24 percent) than in the normobaric oxygen group (43.1 percent).
Strengths
- Well-designed study that included relevant follow up checkpoints with data consisting of both objective (testing) and subjective (self-reported) findings
- Demonstrated a significant different in neurological sequelae in almost all groups
Limitations
- Almost half of those eligible for the study refused randomization (either chose which therapy or did not participate)
- Trial stopped early for benefit
- Control group started with more cerebellar dysfunction (though results still significant after controlled for with regression analysis)
- The study excluded children, pregnant patients
- No assessment of cost-effectiveness
- Reports primary outcome of all neurologic sequelae, but initial endpoint was delayed sequelae (for which there was no difference)
Author's Conclusions
“In summary, treatment of patients with acute, symptomatic carbon monoxide poisoning with three hyperbaric-oxygen sessions within a 24-hour period appears to reduce the rate of cognitive sequelae 6 weeks and 12 months later. Our results support the use of hyperbaric oxygen in patients with acute carbon monoxide poisoning.”
Our Conclusions
It appears that there is a reduction in long term cognitive sequelae with use of hyperbaric oxygen in patients with carbon monoxide poisoning.
Potential Impact To Current Practice
While it is common practice in emergency medicine to treat carbon monoxide poisoning, the verdict is still out on whether or not there is true benefit for hyperbaric therapy over normobaric therapy.
- A Cochrane Review was performed in 2011 that ultimately examined six trials withtwo that found a beneficial effect of HBO for the reduction of neurologic sequelae at one monthandfour others that did find a benefit. As a rersult of this, they released a recommendation that “there is insufficient evidence to support the use of hyperbaric treatment of patients with carbon monoxide poisoning.” (Buckley 2011, PMID: 21491385)
- Following suit, ACEP then released guidelines that state “Emergency physicians should use HBO therapy or high-flow normobaric therapy for acute CO-poisoned patients. It remains unclear whether HBO therapy is superior to normobaric oxygen therapy for improving long-term neurocognitive outcomes.” (https://www.acep.org/patient-care/clinical-policies/carbon-monoxide-poisoning/)
- Our toxicologists tend to recommend that severe cases of CO poisoning be treated with hyperbaric oxygen therapy.
Bottom Line
Hyperbaric oxygen therapy demonstrates a significant reduction of cognitive sequelae at 6 weeks after therapy relative to normobaric oxygen therapy.
Read More
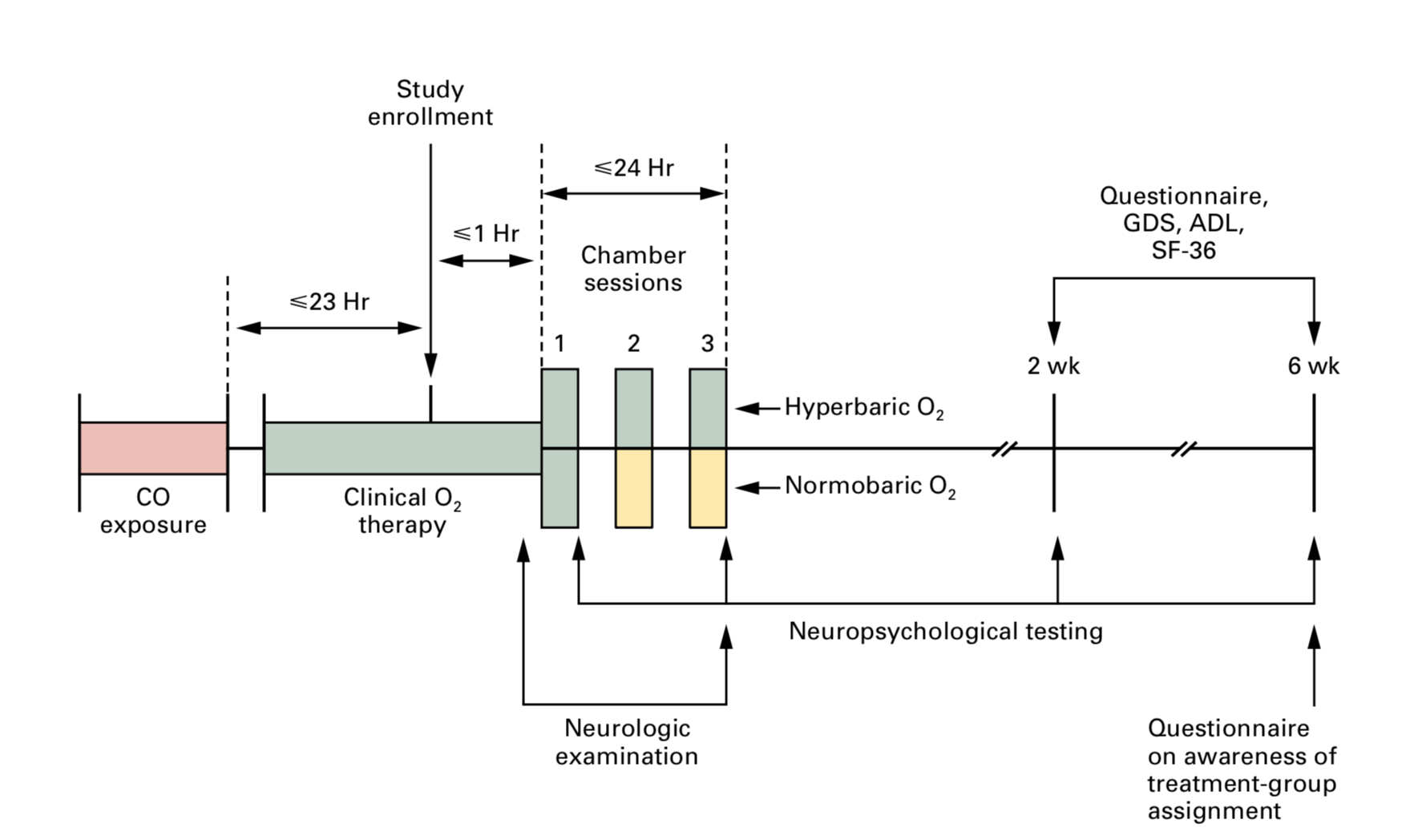
Figure caption from paper: Three chamber sessions were conducted, at intervals of 6 to 12 hours, within a 24-hour period. Neuropsychological testing included tests of general orientation, digit-span, Trail Making Parts A and B, digit–symbol, block design, and story recall. These tests were administered after the first and third chamber sessions and at two and six weeks. Green areas indicate the delivery of oxygen, and yellow areas the delivery of air. GDS denotes Geriatric Depression Scale, ADL the Katz index of activities of daily living, and SF-36 Medical Outcomes Study 36-Item Short-Form General Health Survey.
ALiEM: Carbon Monoxide Poisoning: Common Questions and Dilemmas
ACEP Clinical Policies: Carbon Monoxide Poisoning
References
Buckley NA, Juurlink DN, Isbister G, Bennett MH LE. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev 2011;4 PMID: 21491385
Clardy, P., Manaker, S. and Perry, H. (2019). UpToDate. [online] Uptodate.com. Available at: https://www.uptodate.com/contents/carbon-monoxide-poisoning [Accessed 2 Mar. 2019].
Gozubuyuk AA, Dag H, Kacar A, Karakurt Y, Arica V. Epidemiology, pathophysiology, clinical evaluation, and treatment of carbon monoxide poisoning in child, infant, and fetus. North Clin Istanb. 2017;4(1):100-107. Published 2017 May 10. doi:10.14744/nci.2017.49368 PMID: 28752154
Sircar K, Clower J, Shin MK, Bailey C, King M, Yip F. Carbon monoxide poisoning deaths in the United States, 1999 to 2012. Am J Emerg Med. 2015;33(9):1140-5. PMID: 26032660