Definition: Tissue hypoperfusion that is primarily attributable to damage to the heart.
Criteria: The cardiology literature focuses diagnostic criteria based on systolic blood pressure (SBP) (Gowda 2008)
- SBP < 90 mm Hg
- Decrease in MAP by 30 mm Hg
It is more important, however, to look for evidence of hypoperfusion. In the acute setting, this will typically manifest as a change in mental status (lethargy, decreased responsiveness, agitation, decreased capillary refill, cool extremeties etc.).
Epidemiology:
- Uncommon disorder
- Complicates 7-10% of STEMI
- Complicates 3% of NSTEMI
- Mortality > 80% (Goldberg 1999)
Causes: Acute myocardial infarction (AMI) is the most common cause of cardiogenic shock. Below is a list of other diagnostic considerations that should be entertained:
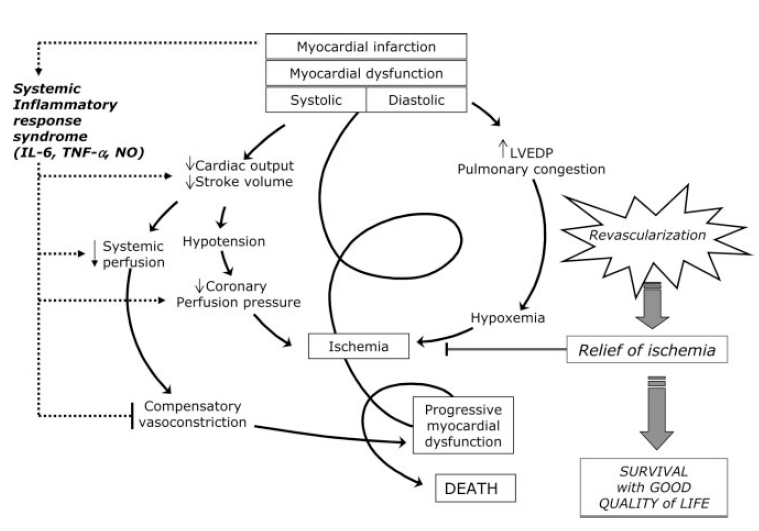
Pathophysiology: This will depend on the causative mechanism. The pathophysiology in AMI induced cardiogenic shock is the most clear:
- AMI leads to LV dysfunction + systemic hypoperfusion
- Systemic hypoperfusion causes neurohormonal activation leading to increased preload and afterload
- Stress on the LV mounts eventually lading to decreased cardiac output
- Decreased cardiac output worsens hypoperfusion and acidemia
- This process spirals creating a vicious cycle:
Signs + Symptoms:
- Shortness of breath
- Dyspnea on exertion
- Diaphoresis
- Cough with pink sputum
- Chest pain
- Air hunger
- Hypoxia
- Tachycardia
- JVD
- Rales
- Skin pallor/mottling
- Altered Mental Status
- Decreased Urine Output
Immediate Management: As STEMI is the most common cause of cardiogenic shock, we will focus on the management of this disorder.
Basics: ABCs, IV, O2, Cardiac Monitor, 12-lead EKG and Ultrasound
Breathing + Circulation
- Severe respiratory distress typically present
- Often will not tolerate non-invasive positive pressure ventilation (NIPPV) and will require emergent intubation
- Challenging intubation secondary to hypotension, hypoxia and acidosis (“HOp killers”)
- Intubation strategy (full discussion beyond scope of this post)
- Maximize preintubation hemodynamics with small fluid bolus + early use of vasoactive substances (push dose pressors)
- Maximize preintubation preoxygenation: high-flow nasal canula + face mask. Consider delayed sequence intubation (DSI) or LMA placement.
- Continue Nasal Oxygenation During Efforts to Secure A Tube (NO DESAT)
- Be wary of worsening hemodynamics during intubation attempts
12-Lead EKG
- AMI is the most common cause of cardiogenic shock and represents one of the fixable etiologies.
- Common EKG patterns leading to cardiogenic shock
- Anterior STEMI (ST elevations in V1-V3)
- Inferior STEMI with extension to the RV (ST elevations in II, III, aVF and right sided lead RV4)
- NB: Look closely for ST elevation in aVR which can represent significant left main coronary artery (LMCA) or left anterior descending (LAD) artery disease.
Chest X-Ray (CXR)
- May be helpful in confirming clinical diagnosis and in ruling out other possible etiologies.
- Most common finding: bilateral pulmonary congestion (INSERT CXR w/ PULMONARY EDEMA)
Point of Care Ultrasound (POCUS)
- POCUS is an important diagnostic modality in patients with suspected cardiogenic shock
- Can aid in narrowing the myriad of diagnoses that can mimic cardiogenic shock
- Common alternate diagnoses: septic shock, massive pulmonary embolism, cardiac tamponade, pneumothorax, severe asthma or COPD exacerbation.
- Additionally, POCUS may identify a ruptured valve causing the patient’s symptoms leading to an alternate management pathway (i.e. cardiovascular surgery for valve repair)
- Read More: US Against the World: Ultrasound in Differentiating COPD from CHF (Boring EM)
Read More: Lichtenstein’s BLUE Protocol
Cardiogenic Shock Diagnostics
Example EKGs, CXR and POCUS in Cardiogenic Shock
Directed Treatment
- Cardiac catheterization
- Immediately activate the cath lab (or arrange transfer to a cath center)
- Emergent successful revascularization can reduce the mortality to less than 50%
- Unsuccessful PCI is associated with an 85% mortality.
- Thrombolytics
- In the absence of a cardiac catheterization lab or significant delay to reach a lab, it is reasonable to consider using systemic thrombolytics.
- However, the SHOCK registry demonstrated no change in outcomes with systemic thrombolytics (Hochman 1995)
- Why thrombolytics don’t work – marginal drug delivery secondary to low diastolic pressure and low coronary artery filling pressure and acidosis.
- Vasoactive Support
- The use of vasopressors should solely be to bridge patients to definitive management (cardiac catheterization, CABG) as they do not fix the underlying physiology
- Ideal agent: increases coronary artery perfusion, minimal effects on heart rate, decreases afterload, decreases myocardial oxygen demand and enhances cerebral perfusion. Unfortunately, no such agent exists.
- ACC/AHA Recommendations (Overgaard 2008)
SBP 70-100 (w/o signs of shock) Start dobutamine SBP 70-100 (w/ signs of shock) Start dopamine SBP < 70 Start norepinephrine ACC/AHA Recommendations based on minimal evidence - Dobutamine (beta-1/2 agonist activity) – may augment cardiac output but also causes vasodilation and may lead to a significant BP drop
- Norepinephrine: Superior to dopamine in undifferentiated shock and in subgroup with cardiogenic shock (De Backer 2010).
- Epinephrine: Viable alternative as increases cardiac contractility and blood pressure but also exacerbates myocardial oxygen demand.
- Intra-Aortic Balloon Pump (IABP)
- Increases coronary artery perfusion and decreases myocardial oxygen demand making in the optimal “pressor” in theory.
- Largest trial to date (IABP-SHOCK II – Thiele 2012): No mortality benefit to IABP placement
Take-Home Points
- Start with what you know. ABCs, IV, O2, Monitor, 12-lead ECG and POCUS.
- Look for a STEMI and activate the cath lab as quickly as possible.
- Address hypotension and hypoxia prior to RSI if possible
- Initiate vasopressor (norepinephrine or epinephrine) and titrate to a MAP of 65 mm Hg.
This post was also featured on REBEL EM here. Thanks to Salim Rezaie and the REBEL EM Crew for supporting the launch of our new site!
References
Gowda RM et al. Cardiogenic Shock: Basics and clinical considerations. Int J Card 2008; 123: 221-228. PMID: 18037513
Goldberg RJ, Samad NA, Yarzdbski J, et al. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N Engl J Med 1999;340:1162–8. PMID: 10202167
Hochman JS, Boland J, Sleeper LA, et al. Current spectrum of cardiogenic shock and effect of early revascularization on mortality: results of an International Registry. SHOCK Registry Investigators. Circulation 1995; 91:873–81. PMID: 7828316
Lichtenstein DA, Meziere GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: The BLUE protocol. Chest 2008; 134: 117-25. PMID: 18403664
Overgaard CB, Dzavik V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation 2008; 118: 1047-56. PMID: 18765387
De Backer D et al. Comparison of dopamine and norepinephrine in the treatment of shock. NEJM 2010; 362: 779-89. PMID: 20200382
Thiele H et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. NEJM 2012; 367: 1287-96. PMID: 22920912